COVID-19 Test Reagents: 12 Chinese companies on WHO’s EUL
COVID-19 Test Reagents: 12 Chinese companies on WHO’s EUL
COVID-19 Test Reagents: 12 Chinese companies on WHO’s EUL . The Emergency Use Listing (EUL) of the World Health Organization (WHO) is to accelerate the availability of in vitro diagnostic products (IVD) required in public health emergencies. It aims to evaluate the quality, safety and performance of IVDs and assist interested purchasing agencies and member states to use specific IVDs.
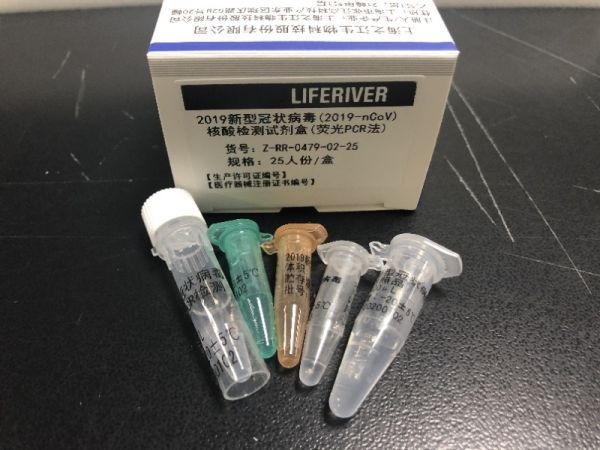
At the beginning of the new coronavirus (COVID-19) pandemic, EUL has opened up in vitro diagnostic products (IVD). On January 30, 2020, the Director-General of the World Health Organization (WHO) Tan Desai announced that the 2019-nCoV epidemic constitutes a public health emergency (PHEIC) of international concern.
By then, the WHO Emergency Use List (EUL) is open to in vitro diagnostic products (IVD) to detect SARS-CoV-2 (originally called 2019-nCoV).
Up to now, only 22 products have passed the COVID-19 EUL list, and the review is very strict. In China, the COVID-19 nucleic acid products of 12 IVD companies have been selected on this list.
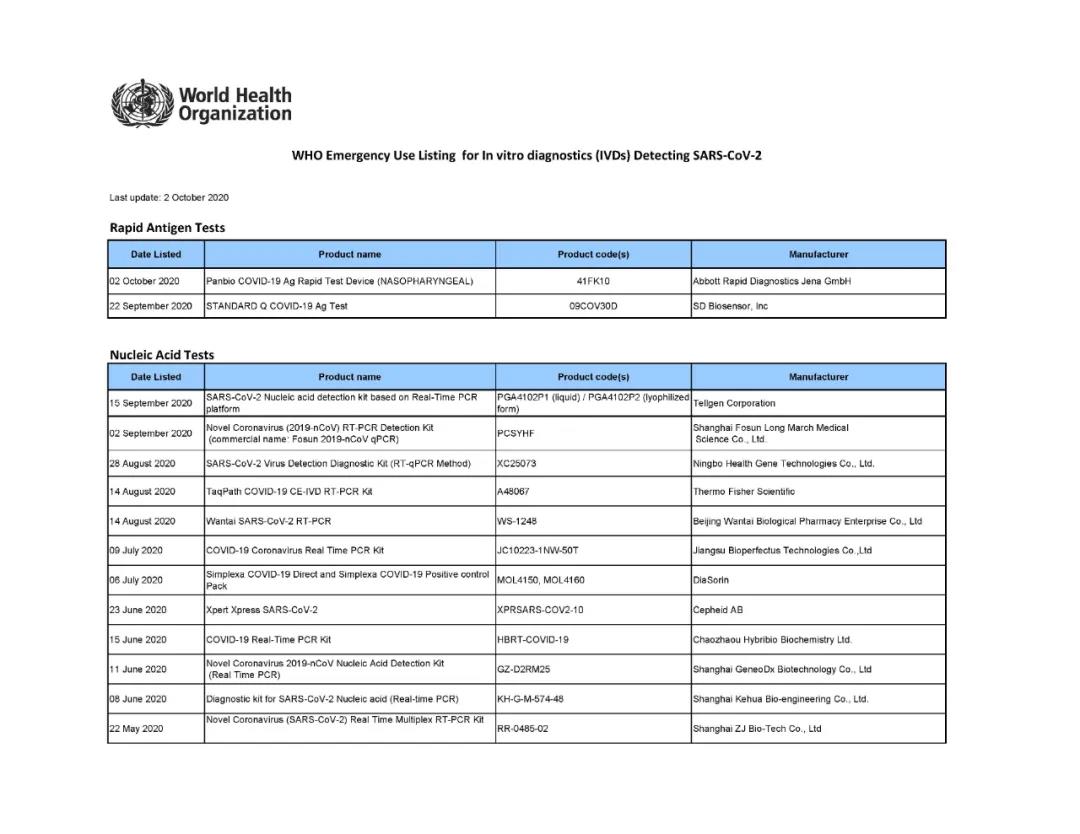
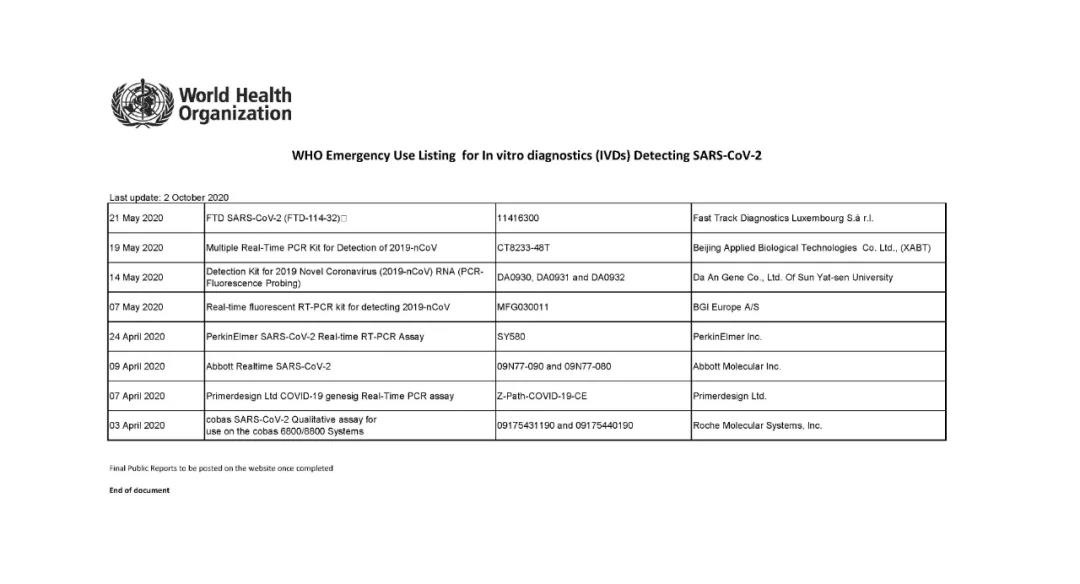
Coronavirus(COVID-19) Reagent Test kits Companies with Certification/Authorization from other Countries
Provided by CCCMHPIE ( China Chamber of Commerce for Import and Export of Medicines and Health Products )



