COVID-19 Vaccine: Kexing launches clinical study of underage in China
COVID-19 Vaccine: Kexing launches clinical study of underage in China
COVID-19 Vaccine: Kexing launches clinical study of underage in China. On December 7, Kexing Holding Biotechnology Co., Ltd. (hereinafter referred to as “Kexing Biotechnology”) announced on its official website that its subsidiary, Beijing Kexing Zhongwei Biotechnology Co., Ltd. (hereinafter “Kexing Zhongwei”), was researching the COVID-19 The phase I/II clinical study of the inactivated vaccine Kellyford™ juvenile group has started. The annual production capacity of Kexing’s COVID-19 vaccine will increase to more than 600 million doses.
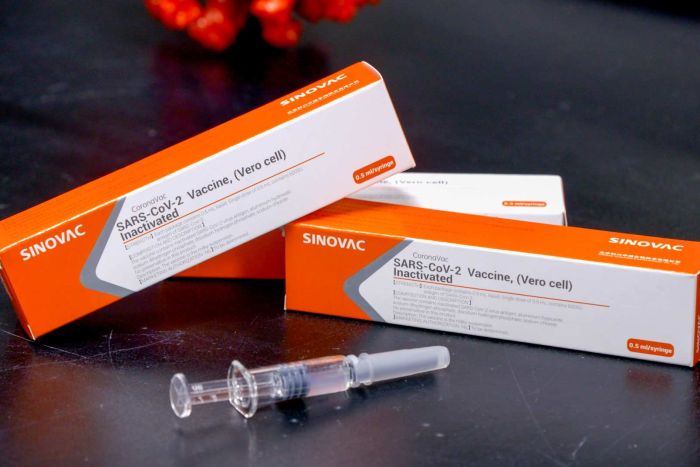
The news shows that Kellyford™️ has obtained the results of phase I/II clinical studies in the adult group and the elderly group in China, and the results of the phase I/II clinical trial for healthy adults aged 18-59 have been released on November 17, 2020. Published online in The Lancet Journal of Infectious Diseases. Phase III clinical studies are being carried out in Brazil, Indonesia, Turkey, Chile and other countries.
According to previous news from Overseas.com, the paper published in the “Lancet Journal of Infectious Diseases” evaluated the results of phase I and phase II clinical trials of the vaccine. The trial involved more than 700 Chinese participants, the most common among participants. The side effect is just the pain at the injection site. The results of the study show that by injecting two doses of the vaccine at an interval of 14 days, Kexing vaccine can quickly induce an immune response within four weeks and is suitable for emergency use during the COVID-19 pneumonia pandemic.
The article said that to determine whether the immune response triggered by the vaccine can protect people from infection, the results of large-scale late-stage trials, that is, phase III clinical trials, are particularly important, and the duration of the antibody response must be studied in the future.
According to the official website of Kexing Biology, Kexing Zhongwei began to pay attention to the COVID-19 epidemic in early January 2020 and began various preliminary preparations for vaccine development, and then officially launched a new type of coronavirus called “Crown Action” on January 28 Vaccine development project. The project was approved for clinical use on April 13, 2020, and the adult group (18 to 59 years old) Phase I/II clinical study was subsequently launched in Jiangsu on April 16. The project is supported by the Ministry of Science and Technology “National Key R&D Program”, the Beijing Municipal Science and Technology Commission “City Science and Technology Program” project and the production conditions of Daxing District.
In June 2020, the blind review and unblinding meeting of the COVID-19 inactivated vaccine Kellyf™️ Phase I/II clinical study (0,14 procedures) was held in Beijing. A total of 743 subjects in phase I/II clinical studies have all completed vaccination. Phase II clinical study 0,14 days program immunogenicity results showed that the positive conversion rate of neutralizing antibody after 14 days of full immunization exceeded 90%, indicating that the vaccine has good immunogenicity.
It is worth mentioning that the US pharmaceutical company Pfizer has recruited minors into its clinical trials of the COVID-19 vaccine under development, and the US pharmaceutical company Moderna also has related plans.
Pfizer announced on its official website that in October 2020, it received the U.S. Food and Drug Administration (FDA) permission to recruit young people as young as 12 years old into clinical trials of its new coronavirus mRNA vaccine. Pfizer said that in this way, the company can better understand the potential safety and effectiveness of the vaccine in people of more ages and backgrounds.
According to a report from the New York Times on December 2 local time, Moderna said on Wednesday that he would soon carry out his research on the COVID-19 vaccine among minors aged 12 to 17.
The study was published on clinicalTrials.gov on December 2, local time.
clinicalTrials.gov is a resource provided by the US National Library of Medicine. Research published on this website does not mean that it has been evaluated by the US federal government.
According to Moderna’s announcement, the name of the study is “a phase 2/3 randomized, observer-blinded, placebo-controlled trial to evaluate the safety of mRNA-1273 Covid-19 vaccine for healthy adolescents aged 12 to 18 years. Reactogenicity and effectiveness.”
The study is expected to recruit 3,000 people into the group, and the mRNA-1273 vaccine will be given to adolescents with two doses 28 days apart. Currently, recruitment for this study has not yet begun.
Previously, on December 30, local time, Moderna announced that the effective rate of its new coronavirus vaccine mRNA-1273 under development was 94.1%, and the effective rate for preventing severe COVID-19 pneumonia was 100%. The data is based on its Phase III clinical study, and the participants are all 18 years old or older.
Pfizer Phase III data was released earlier. Previously, on November 18, local time, Pfizer announced on its official website that the mRNA COVID-19 vaccine candidate BNT162b2 developed in cooperation with the German BioNTech company has an effective rate of 95% 28 days after the first administration. On November 20, local time, Pfizer and BioNTech submitted an emergency use authorization application to the US FDA. The submitted report included safety data on approximately 100 children aged 12-15.
According to a report in late October, Dr. Robert Frenck Jr., director of the Vaccine Research Center at Cincinnati Children’s Hospital, said that at least 500,000 U.S. minors have been infected with the COVID-19 pneumonia this year, and there may be more. The extent to which you need to see a doctor”.
Disclaimer of medicaltrend.org



