FDA: No clear evidence of vaccines related on 4 volunteers with facial paralysis
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
FDA: No clear evidence of vaccines related on 4 volunteers with facial paralysis
FDA: No clear evidence of vaccines related on 4 volunteers with facial paralysis. Pfizer 4COVID-19 vaccine volunteers developed facial paralysis, FDA: no clear evidence of COVID-19 vaccine related.
On December 8, the US Food and Drug Administration (FDA) issued a report stating that in the Phase III clinical trial of Pfizer vaccine, 4 subjects in the experimental group developed idiopathic facial nerve palsy (Bell’s palsy). No one in the placebo group had this symptom.
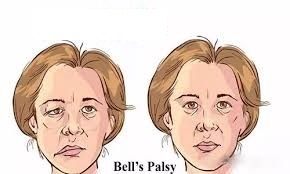
The FDA report believes that there is no clear evidence to prove that there is a causal relationship between the two. “The proportion of 4 Bell’s facial paralysis cases in the injection group is not higher than the incidence of the disease in the general population”, but it is still recommended to deploy the vaccine to more people At the same time, cases of idiopathic facial nerve palsy were monitored.
The effectiveness of two doses of the vaccine was 95.0%. There were 8 cases of COVID-19 in the vaccine group and 162 cases of COVID-19 in the placebo group. The report shows that the 95% confidence interval for vaccine effectiveness is 90.3% to 97.6%.
The FDA still believes that Pfizer vaccines are safe and effective. Among the participants in the clinical trial of Pfizer vaccine, 56% of the subjects experienced fatigue symptoms after the injection, 47% of the subjects had headache symptoms, 31% of the subjects had chills, 21% of the subjects had joint pain, and 14% Participants developed fever symptoms.
Disclaimer of medicaltrend.org



