First Inactivated COVID-19 Vaccine Approved
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
First Inactivated COVID-19 Vaccine Approved
First Inactivated COVID-19 Vaccine Launched in China. China State Council’s joint prevention and control mechanism announced at the press conference that the COVID-19 inactivated vaccine of the Beijing Institute of Biological Products of SINOPHARM Group has been approved by the State Food and Drug Administration for conditional listing.
According to the information at the press conference, the protection rate of the vaccine is 79.34%, which meets the relevant standards of the World Health Organization and the National Food and Drug Administration. The durability of subsequent vaccine immunity needs to be continuously observed.
In terms of prices, Zeng Yi, deputy director of the National Health Commission and head of the vaccine research and development team of the Joint Prevention and Control Mechanism of the State Council, said: “The basic attributes of vaccines are still public products, and prices may vary according to the scale of use. . But a major premise is that it must be provided free of charge for all people.”
Source: Sinopharm Beijing Institute of Biological Products Co., Ltd.
According to reports, by the end of November, a total of more than 1.5 million doses of the vaccine had been vaccinated, and about 60,000 of them went to work in high-risk areas overseas, and there were no reports of serious infections. China officially launched the vaccination of key populations in China on December 15. In the past two months, the cumulative vaccination of key populations across the country has exceeded 3 million doses.
It is understood that the overall incidence of adverse reactions of this vaccine is close to that of conventional inactivated vaccines, with less than 0.1% of mild fever cases and 2 per million cases with more serious allergies.
According to the vaccine instructions:
- This product must be used in accordance with the national immunization plan and related immunization strategies, and under the guidance of the national health authority and relevant disease control management agencies.
- Usage: The recommended route of vaccination is intramuscular injection. The best part is the deltoid muscle of the upper arm.
- Dosage: The basic immunization is 2 doses with an interval of 2 to 4 weeks. 0.5 ml each time.
- Indications: This product is used for adults and children over 3 years old, especially medical personnel and other close contacts.
- Vaccination of this product can stimulate the body to produce immunity against the new type of coronavirus, which is used to prevent diseases caused by the new type of coronavirus. (Click to view the complete instructions for the COVID-19 vaccine)
Inactivated vaccine technology
What kind of technology is inactivated vaccine? The first is to select a suitable virus strain for vaccine production. Sinopharm Group selected a new coronavirus isolated from a patient in Jinyintan Hospital.
The second is to select suitable virus-cultured cells. Based on Vero cells that have previously established a large-scale production system, they are used to cultivate new coronaviruses and provide large-scale viruses for the inactivation process.
The third is to choose a reliable inactivation process. β-propiolactone is the second reagent used for virus inactivation after formaldehyde. Current studies believe that β-propiolactone changes the nucleic acid structure of the virus through alkylation, so that the virus loses the ability to replicate, thereby achieving the purpose of inactivation.
The fourth is to design an efficient purification process. After the virus is cultured and inactivated, there are fragments and DNA from Vero cells that need to be purified to improve the purity of the vaccine, which is also the guarantee of vaccine safety.
Finally, the adjuvant is used to adsorb the inactivated virus to improve the immunogenicity of the vaccine. Aluminum hydroxide adjuvant is the most classic vaccine adjuvant and is widely used in a variety of vaccines.
Inactivated vaccine technology is a relatively mature vaccine technology route. At present, common vaccines that use the same technical route include: inactivated polio vaccine, inactivated hepatitis A vaccine, rabies vaccine, and EV71 inactivated vaccine.
Past paper data
This vaccine has previously passed phase I/II clinical trials and published an article disclosing phase I/II trial data.
This vaccine will start a phase I clinical trial in Liang Park, Shangqiu City, Henan Province in April 2020.
A total of 192 people were enrolled in the phase I trial, divided into 18-59 years old group and ≥60 years old group. Each group was divided into 4 groups and received low, medium and high dose vaccine or placebo on day 0/28. .
Among the vaccinated subjects, 42 people (29%) had adverse reactions: among the 18-59 year-old volunteers, 11 people (46%) and 8 people (24%) were in the low, medium, and high dose groups, respectively. ), 11 people (46%) had adverse reactions; 1 person (4%), 6 people (25%), and 5 people (21%) in the middle, low, middle, and high dose groups ≥60 years old had adverse reactions.
The most common adverse reactions are local reactions such as pain at the injection site. The most common systemic adverse reaction was fever. Fever occurred in 1, 1, and 2 volunteers in the low, medium, and high dose groups.
During the trial period, no volunteers experienced serious adverse reactions related to vaccination. These adverse reactions are self-limiting and can subside on their own without treatment.
28 days after receiving the first dose of vaccine, 100% of the volunteers in the vaccine group aged 18 to 59 were able to detect neutralizing antibodies. Among volunteers ≥60 years old, 91% of the low-dose vaccine group, 92% of the medium-dose vaccine group, Ninety-six percent of subjects in the high-dose vaccine group could detect neutralizing antibodies. On the 42nd day of the second vaccination, all subjects in the vaccine group were able to detect neutralizing antibodies.
Based on the results of the phase I trial, the phase II trial will focus on “two medium-dose vaccines” and “a single high-dose vaccine.”
Phase II clinical trials continued in Henan, with a total of 448 volunteers divided into a control group and a vaccine group. Slightly different from the phase I trial, subjects in the vaccine group were divided into four groups: receiving a single high-dose vaccine on day 0, or receiving two vaccines on day 0/14, 0/21, and 0/28. Medium dose vaccine.
- Among the subjects in the phase II trial, 76 people (23%) in the vaccine group experienced adverse reactions.
- Except for a case of hyperthermia that resolved spontaneously in the control group, the other adverse reactions were similar to those in the phase I trial.
- The entire phase II clinical trial was the same as the phase I trial, and there were no serious adverse reactions related to vaccination, showing that the vaccine is very safe.
- In the phase II trial, regardless of the dose and time of vaccination, all subjects in the vaccine group developed neutralizing antibodies 14 days after the last vaccination.
- The antibody titers (14.7) of subjects receiving a single high-dose vaccine 28 days after vaccination were lower than the antibody titers of the other groups at 28 days after the second vaccination, and it was statistically significant. This means that when the total dose is the same, a single high-dose vaccine is less effective than two separate injections of the medium-dose vaccine.
- The antibody titers of the 0/14 day group at 28 days of the second vaccination were lower than the 0/21 and 0/28 groups, which were 169.5, 282.7 and 218.0, respectively, and were statistically significant. This means that the interval between the two vaccines needs to be explored in phase III trials, and the specific results will be disclosed in the near future.
Effectiveness
In mid-July, Sinopharm Vaccine conducted a cross-neutralization test against the possible mutations of the COVID-19 virus from Beijing Xinfadi, Russia, the United Kingdom, Austria, the United States and other places with the COVID-19 inactivated vaccine, and the results showed that all can be 100% neutralize.
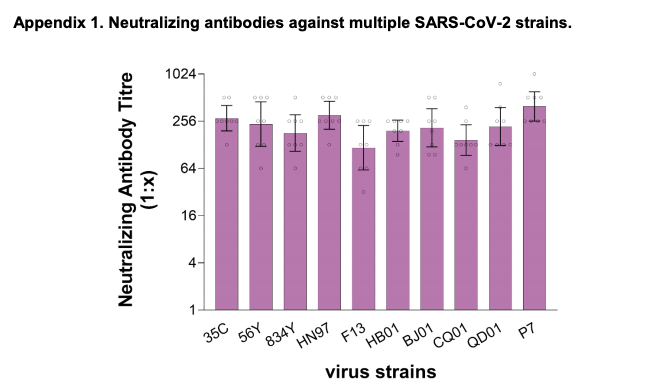
Picture source: reference 4
This result suggests that although the new coronavirus is indeed mutating, its main antigen protein sequence or most of the neutralizing antibody epitopes should not be fundamentally changed, and the vaccine can now cover these mutated viruses better.
In addition, this result also suggests that the existing vaccines are also expected to still have good protective effects for new virus epidemic strains that have significantly increased infectivity in the near future.
At the State Council’s joint prevention and control press conference held today, the Ministry of Science and Technology stated that the scientific research team has held multiple meetings. Experts believe that there is no evidence that the new coronavirus variant will have a substantial impact on the effectiveness of the vaccine.
Other vaccine progress
At the State Council Joint Prevention and Control Press Conference, Xu Nanping, Vice Minister of Science and Technology, stated that with the approval of the Beijing Institute of National Medicines vaccines, China has first 5 technical routes and 14 vaccines to enter clinical trials, including 3 technical routes and 4 vaccines. The vaccine is in the mid or late phase of Phase III clinical trials.
Acknowledgements: This article has been professionally reviewed by a Ph.D. in Microbiology, Chinese Academy of Sciences
【Note】PhD in Microbiology, Chinese Academy of Science Review Opinion:
The COVID-19 inactivated vaccine of the Beijing Institute of Biological Products of Sinopharm Group was quickly approved with a protection rate of 79.34%, which is an excellent milestone in China’s fight against the COVID-19 epidemic.
This vaccine is the earliest and fastest vaccine developed in the country. However, judging from the existing published preclinical and clinical phase I/II data, the country has not relaxed the standard for the quality of the vaccine and the scientific design of clinical trials. China’s first inactivated COVID-19 vaccine shows excellent safety, a very high neutralizing antibody positive conversion rate and a protection rate that is not weaker than that of the existing approved COVID-19 vaccine.
Although there are no complete data disclosures, especially for some groups of people (such as the elderly, patients with underlying diseases, immunodeficiency, young people, etc.), there are no specific data and guidance for immunization opinions, but please be patient and trust the country , In the end, it will give everyone a satisfactory answer.
China currently has at least four COVID-19 vaccine candidates in clinical phase III, some of which are close to the clinical endpoint. I believe that in the next few months, our country will continue to have more candidate COVID-19 vaccines for domestic use in China. Conditionally support international anti-epidemic needs.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



