Hepatitis B therapy: Agreement between Gilead and Vir
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
- Engineered Soybeans with Pig Protein: A Promising Alternative or Pandora’s Dish?
- Severe Fever with Thrombocytopenia Syndrome (SFTS): A Tick-Borne Threat with High Mortality
Hepatitis B therapy: Agreement between Gilead and Vir
Hepatitis B therapy: Agreement between Gilead and Vir. Gilead and Vir have reached a partnership to explore functional hepatitis B combined therapy.
Both companies retain all rights to their respective drug candidates and will discuss the future development direction of combination therapies based on the results of phase 2 clinical trials.
On January 12, 2021, Gilead and Vir Biotechnology announced that the two companies have reached a clinical collaboration to develop innovative therapeutic combinations that can functionally cure chronic hepatitis B virus (HBV).

HBV affects more than 290 million people worldwide. Globally, HBV is the main cause of liver cancer; it is estimated that 800,000 people die from HBV-related liver diseases every year. Although current antiviral therapies can continue to suppress the HBV virus, they rarely completely eliminate the virus. Therefore, HBV patients require life-long treatment.
The two parties plan to start a phase 2 clinical trial of combination therapy for treated and untreated HBV patients. This multi-arm trial will evaluate different combinations of selgantolimod, VIR-2218, and a PD-1 antagonist that is already on the market. Selgantolimod is a TLR-8 agonist developed by Gilead, and VIR-2218 is a siRNA drug from Vir.
HBV patients who have been treated in the trial may also take Gilead’s Vemlidy® (tenofovir fumarate, TAF). The primary endpoint of the study was the proportion of patients who achieved functional cure, defined as the undetectable hepatitis B surface antigen (HBsAg) and HBV DNA in the serum after stopping treatment.
Both companies retain all rights to their respective drug candidates and will discuss the future development direction of combination therapies based on the results of phase 2 clinical trials. However, selgantolimod and VIR-2218 are still in the research and development stage and have not been approved by any regulatory agency. Therefore, the safety and effectiveness of the two drugs cannot be determined.
Gilead’s selgantolimod (GS-9688) is an oral selective TLR8 agonist. Data from a phase 2 clinical trial (NCT03615066; GS-US-389-2025) published in November last year showed that at week 24, no patient reached the primary endpoint of HBsAg drop ≥1 log10 IU/ml, but all treatment groups HBsAg drop <0.1, ≥0.1- <0.3 and ≥0.3- <1.0 log10 IU/ml, HBsAg drop ≥0.3 log10IU/ml only appeared in the selgantolimod treatment group, and no patients lost HBsAg or HBeAg at week 24.
The proportion of HBV DNA <20 IU/ml decreased in all treatment groups was similar. In viral suppression (VS) and viremia patients with chronic hepatitis B, selgantolimod cytokine responses are similar. Selgantolimod dose-dependently induced the cytokine IL-12p40, IL-1 receptor antagonist and IFNγ in patients with VS and viremia.
The original research company of VIR-2218 (ALN-HBV-02) was Alnylam, and VirBiotechnology was authorized. This is a siRNA drug designed to use Alnylam’s enhanced stabilization chemical (ESC+)-GalNAc conjugate technology to be administered by subcutaneous injection to effectively silence RNA transcripts necessary for HBV virus replication and protein expression, thereby treating chronic HBV infection .
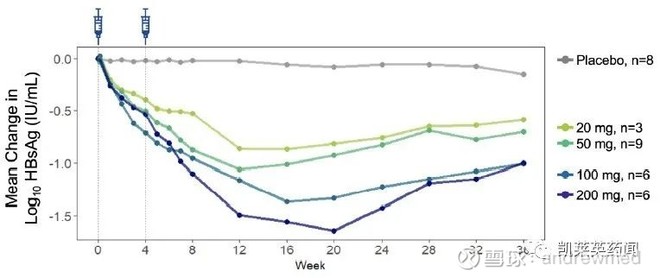
According to the results of a recently announced phase 2 clinical trial (NCT03672188; VIR-2218-1001), in patients with chronic HBV, VIR-2218 caused a dose-dependent and persistent reduction of hepatitis B surface antigen (HBsAg) within 36 weeks. Most of the treatment-related adverse events were mild.
(sourcechinanet, reference only)
Disclaimer of medicaltrend.org



