Nature: The new regulatory protein iPLA2β for iron death
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
Nature: The new regulatory protein iPLA2β for iron death
Nature: The new regulatory protein iPLA2β for iron death. Nature Sub-Journal: Reversing iron death, treating Parkinson’s, He Rongrong’s team and others reported on the new regulatory protein iPLA2β for iron death.
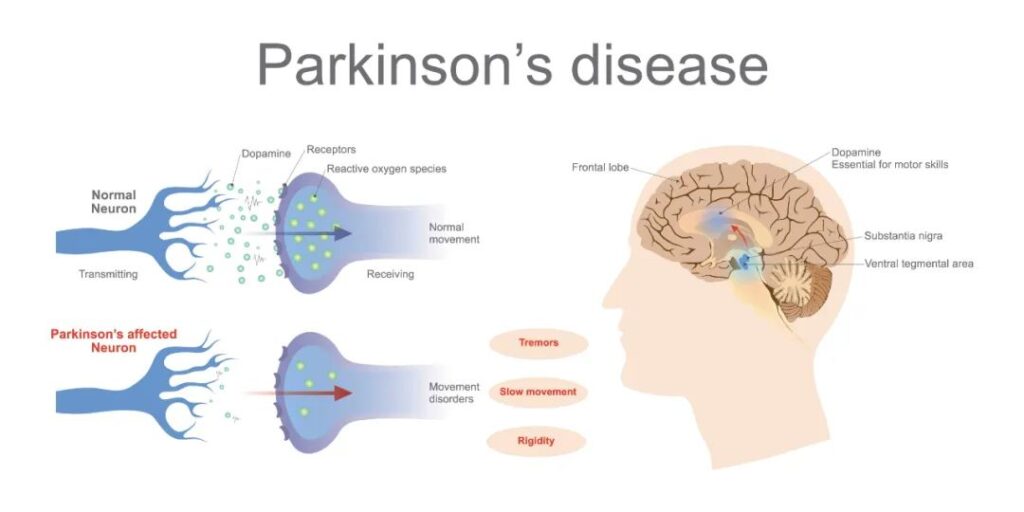
This study mainly revealed that the oxidative accumulation of phospholipids in dopaminergic neurons is an important pathological mechanism of Parkinson’s disease.
The degenerative death of dopaminergic neurons is the main pathological mechanism leading to PD in the clinic. Current research suggests that apoptosis, necroptosis, and autophagic death are all involved in the degenerative loss of dopaminergic neurons.
However, these death modes are not enough to explain the pathological process and mechanism. In-depth research has found that these death modes have a common feature, that is, the lipid peroxidation damage of dopaminergic neurons. The cell death method that can truly reflect the lipid peroxidation of cells is the newly discovered cell death method in recent years-iron death.
The main characteristics of iron death are the increase of iron ion load as the driving factor and a large amount of lipid peroxide as the cell death factor, and these characteristics are highly consistent with the clinical molecular biological characteristics of the brain changes in Parkinson’s patients.
The team of Professor Rongrong He from Jinan University has long been concerned about the impact of emotional stress-induced cellular oxidative stress damage on the “susceptibility” of Parkinson’s disease, and proposed that the oxidation of dopaminergic neuron phospholipids may be an important pathological mechanism of Parkinson’s disease.
Professor Kagan’s research group has previously discovered that 15-LOX can be anchored to the cell membrane under the action of the partner molecule PEBP1, and specifically oxidize phosphatidylethanolamine (PE) to form oxidized phosphatidylethanolamine (ox-PE), of which the PE oxidation product 15 -HpETE-PE is an important signaling molecule for iron death in cells (Nat Chem Biol. 2017, 13:81-90; Cell. 2017, 171:628-641). In the process of cooperation, the two parties confirmed that the oxidation of dopaminergic neuron phospholipids is involved in the pathological process of Parkinson’s, and the mechanism is related to the ineffective repair of oxidized phospholipids.
Unsaturated fatty acid chains of phospholipids in biological membranes are constantly oxidized and repaired. If the repair is not timely, a large accumulation of phospholipid peroxides may cause iron death of cells. In the process of oxidized phospholipid repair, calcium-independent phospholipase A2β (iPLA2β, the corresponding gene name is PNPLA9) plays an important role. iPLA2β is a protein that specifically hydrolyzes the sn-2 acyl bond of phospholipids, and plays an important role in the removal of oxidized phospholipids from cell membranes and the remodeling of phospholipids.
Clinical studies have shown that PNPLA9 mutation is closely related to the pathogenesis of Parkinson’s disease, but its pathogenesis is unclear. More than 80 different PNPLA9 mutation types have been found in clinical practice. In this study, the author focused on the pathogenesis of PNPLA9R747W mutation type, and the conclusions obtained are also applicable to other mutation types.
Recently, the team of Professor Rongrong He of Jinan University and the team of Professor Valerin E. Kagan of the University of Pittsburgh jointly published a research paper entitled Phospholipase iPLA2β Averts Ferroptosis By Eliminating A Redox Lipid Death Signal in Nature Chemical Biology.
The study used oxidized lipidomics and other technologies to clarify that the mutation of phospholipid remodeling key protein iPLA2β leads to a decrease in the activity of its hydrolyzed oxidized phospholipid ox-PE, leading to the accumulation of oxidized phospholipid in dopaminergic neurons.
The study found that iPLA2β is an important regulator of iron death. The accumulation of lipid peroxides caused by the loss of its activity is closely related to the occurrence of Parkinson’s disease. Inhibiting iron death is expected to become a new strategy for the treatment of Parkinson’s disease.
In this study, the researchers started from the clinical phenomenon (iPLA2β mutant Parkinson’s disease patients have obvious accumulation of oxidized phospholipids), using healthy human cells H109 and iPLA2β mutation Parkinson’s disease cells fPDR747W to extract cellular phospholipids for oxidation Phospholipid omics experiment. Experimental results show that compared with normal human cells H109, fPDR747W cells have a significantly higher phospholipid peroxidation level, and the iron death oxidized phospholipid signaling molecule 15-HpETE-PE level is also significantly higher.
To further explore the relationship between iPLA2β deficiency and iron death, the authors administered RSL3 to H109 and fPDR747W cells respectively to observe the changes in cell viability. When iPLA2β function is lost, cells are more sensitive to iron death caused by RSL3. This phenomenon has also been observed in iPLA2β protein knockdown and knockout cells, indicating that iPLA2β can inhibit iron death of cells to a certain extent.
Professor Rongrong He’s team used CRISPR-Cas9 technology to construct Pnpla9R748W point mutation mice (corresponding to human R747W mutation site) to verify the relationship between iPLA2β function and the pathogenesis of Parkinson’s disease.
Compared with WT mice, homozygous mutant mice began to appear climbing rods, turning rods, and gait behavior abnormalities at the age of 3 months (compared with the commonly used SncaA53T mouse model of Parkinson’s disease, the onset time of this model was significantly shorter); The levels of dopamine and its metabolites in the brain of 7-month-old homozygous mutant mice were significantly reduced, 4-HNE levels were significantly increased, GSH was depleted, and the levels of iron death signaling molecule 15-HpETE-PE were significantly increased.
In vitro enzyme activity experiments showed that the expression level of iPLA2β in the midbrain of mutant mice was unchanged, but the catalytic activity was significantly reduced.
In order to explore the relationship between phospholipid peroxidation and the pathogenesis of Parkinson’s disease, the researchers further established a murine model of Parkinson’s disease induced by rotenone and SCNA-A53T. The oxidized lipidomics analysis of the substantia nigra of rats induced by rotenone showed that ox-PE increased significantly.
When rotenone was continuously administered for 10-14 days, 15-HpETE-PE accumulated in the substantia nigra of rats, and iPLA2β activity was significantly reduced. The 8-month-old SncaA53T mouse model was studied, and it was found that 15-HpETE-PE accumulated in the midbrain of mutant mice, and the expression of iPLA2β was significantly reduced. The above results indicate that the accumulation of lipid peroxides caused by the loss of iPLA2β function also exists in other Parkinson’s disease models.
Using iPLA2β WT and R747W to catalyze the two substrates of non-oxidized phospholipid 1-SA-2-ETE-PE and oxidized phospholipid 1-SA-2-15-HpETE-PE in vitro, it was found that iPLA2β has a positive effect on 1-SA-2 The catalytic activity of -15-HpETE-PE is higher than that of 1-SA-2-ETE-PE, and when iPLA2β point mutation, the catalytic activity of this enzyme on 1-SA-2-15-HpETE-PE is greatly reduced. Similar results were also observed in fPDR747W mutant skin fibroblasts of patients with Parkinson’s disease.
In order to understand the mechanism by which mutations lead to the loss of iPLA2β function, computer simulations of the interaction of iPLA2β WT and R747W dimers with cell membrane phospholipids were further used (the figure below). After the wild-type protein is anchored to the cell membrane, the phospholipid easily enters the active pocket and is close to the active center site, which is beneficial to the enzyme-catalyzed reaction. After the protein is mutated, the active pocket is reduced, and the phospholipid is difficult to access the active center site, which hinders the enzymatic reaction. In addition, the mutant protein also produced a new binding conformation without catalytic activity.
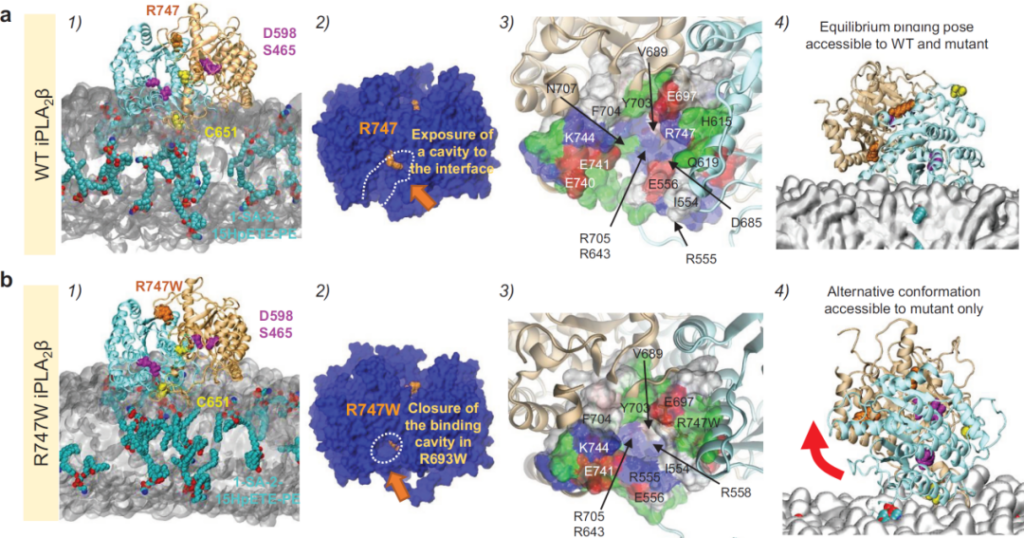
The mutation causes the conformational change of iPLA2β and cell membrane phospholipid binding, and the ability to enzymatically degrade oxidized phospholipid is reduced.
This study mainly reveals that the oxidative accumulation of dopaminergic neuron phospholipids is an important pathological mechanism of Parkinson’s disease, and proves that the loss of iPLA2β function can lead to the “susceptibility” of dopaminergic neuron iron death, and the anti-iron death function of iPLA2β is mainly to eliminate iron death. The death signaling molecule 1-SA-2-15-HpETE-PE.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



