Cytokine pathway (JAK-STAT pathway)
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
- Engineered Soybeans with Pig Protein: A Promising Alternative or Pandora’s Dish?
- Severe Fever with Thrombocytopenia Syndrome (SFTS): A Tick-Borne Threat with High Mortality
Cytokine pathway (JAK-STAT pathway)
Cytokine pathway (JAK-STAT pathway). The extracellular domains of RTKs can identify growth factors such as EGF and IGF, while the intracellular domains have tyrosine kinase domains (TK domains). The type of receptor to be introduced today is quite special: its extracellular domain can recognize signals such as cytokines, but its intracellular domain does not have a TK domain, but has a binding site for the intracellular tyrosine kinase JAK. Intracellular signals are further transmitted through JAK kinase and downstream protein STAT. This type of pathway is collectively referred to as the cytokine pathway, also known as the “JAK-STAT pathway”.
01 Cytokines and cytokine receptors
Broadly speaking, cytokines include growth factors, TNF-related factors, chemokines, and many other signal factors, but the cytokines we are going to discuss here only refer to the ability to recognize receptors that do not have intracellular TK domains but have JAK binding sites. The ligand.
There are about 40 such cytokines, which can be divided into two categories as shown in the table below. Among them, the first category can be divided into the following families, which are characterized by having 4 α-helices in structure:
- Interleukin-2 (IL2) family: IL2, IL4, IL7, IL9, IL15 and IL21.
- Interleukin-6 (IL6) family: IL6, IL11, IL27, LIF, CNTF and OSM.
- Interleukin-3 (IL3) family: IL3, IL5 and GMCSF.
- Interleukin-12 (IL12) family: IL12 and IL23.
- Hormones and other hematopoietic growth factor families: GCSF, EPO, TPO, GH, prolactin and leptin.
The second category includes: interferon and other interleukin subfamily, IL10 family (IL10, IL22, IL26) and IL20 family (IL19, IL20, IL24).
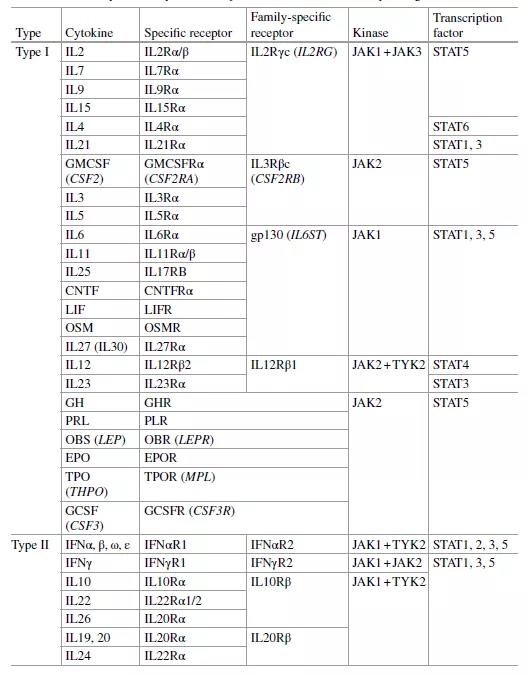
Table 1. Cytokines and cytokine receptors
The cytokine receptor does not have a kinase catalytic domain in the cell, but has several relatively conserved structures. Among them, a sequence near the cell membrane—sequence box 1 (Box1) is composed of 8 prolines; the other—sequence box 2 (Box2) is composed of hydrophobic amino acids, followed by a series of charged amino acids and Phosphorylated tyrosine residues. Studies have shown that these two conserved structures are extremely important for the binding of JAK. What is more special is that CNTF receptors have no transmembrane and intracellular domains, but are anchored to the plasma membrane through a glycosylphosphatidylinositol molecule.
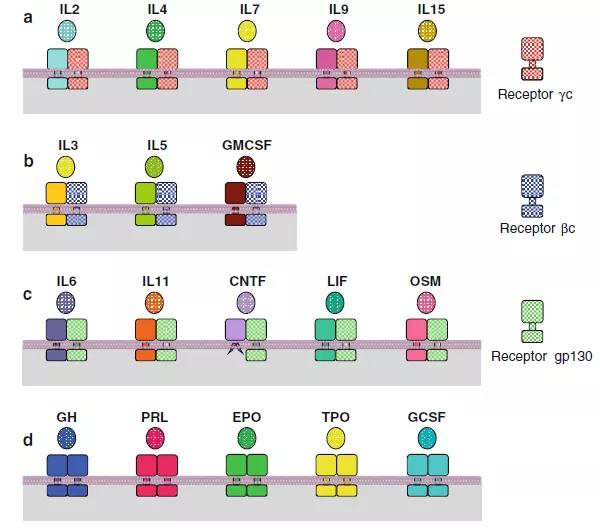
Figure 1. The signal-receiving complex of major type I cytokines. (a) Alpha receptor and γc receptor heterodimerization. (b) Alpha receptor and βc receptor heterodimerization. (c) Alpha receptor and gp130 receptor heterodimerization. (d) Homodimerization.
Similar to RTKs, cytokine receptors also need to dimerize to activate downstream pathways. After the cytokine is combined with the corresponding specific receptor monomer (α type), it will trigger its dimerization with another family specific receptor monomer (for example: γc, βc, gp130, etc.), the specific combination The way is shown in Figure 1 and Table 1.
02 Cytokine receptors and JAK activation
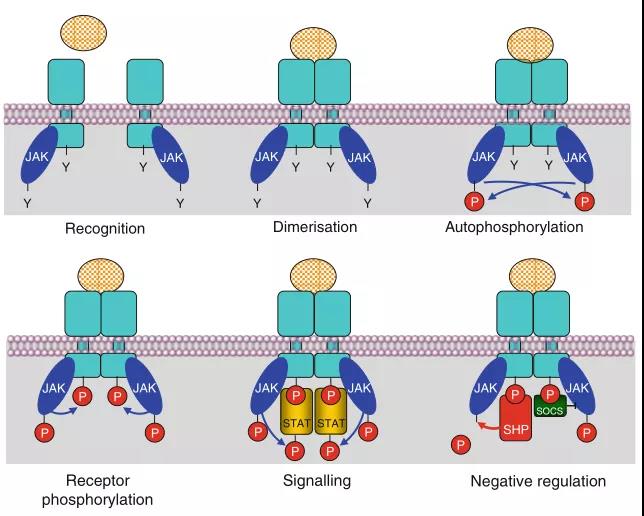
Figure 2. Schematic diagram of cytokine receptors activated by JAK kinase
As mentioned above, cytokine receptors must be homodimerized or heterodimerized to activate the signaling pathway (Figure 1). After the dimer and its ligand constitute the “receiving complex” to be transmitted, the JAK kinase in the cell is activated to autophosphorylate (Figure 2). After autophosphorylation, JAK kinase phosphorylates receptor tyrosine residues. Four JAK proteins have been identified, namely JAK1, JAK2, JAK3 and TYK2. The correlation between different JAK proteins and receptors is shown in Table 1. After autophosphorylation and receptor phosphorylation occur, STAT family transcription factors with SH2 domain (mentioned in the first issue: able to recognize phosphorylated tyrosine on the receptor) are recruited to the membrane and phosphorylated by JAK化.
Although different JAK proteins overlap in function, they may play a corresponding role in different tissues. JAK1, JAK2 and TYK2 are widely distributed in the body, while JAK3 is specifically expressed in hematopoietic tissues and is activated by IL2 family cytokines; JAK1 can be activated by many cytokines, especially IL2, IL6 and type II cytokines; JAK2 It produces specific responses to hematopoietic factors (EPO) and hormones; and TYK2 is mainly involved in type II cytokine responses.
03 JAK-STAT pathway
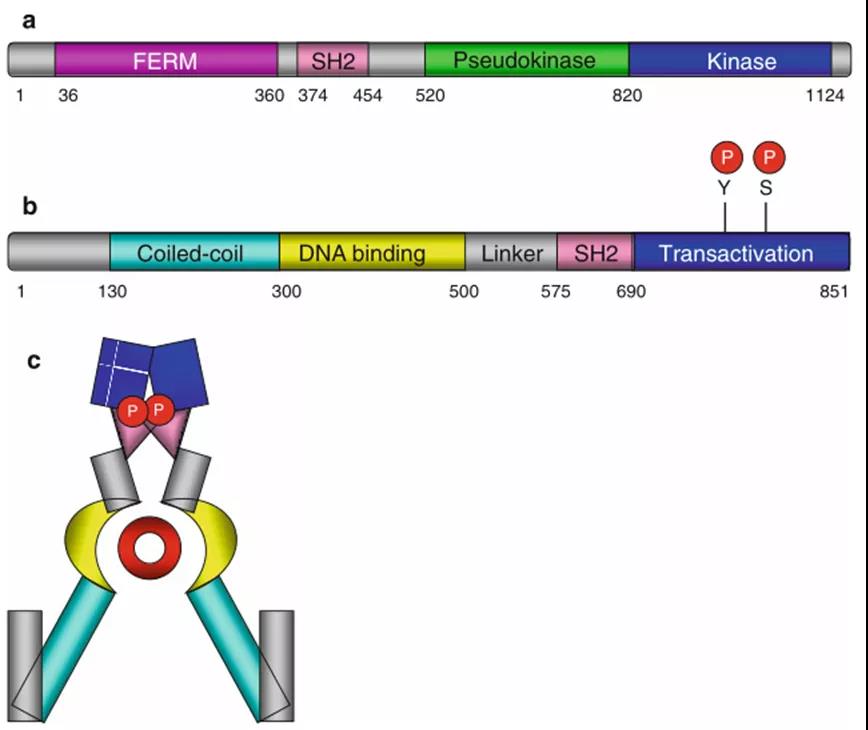
Figure 3. General structure of JAK and STAT3. (a) General structure of JAK protein. (b) General structure of STAT protein. (c) Dimerized STAT protein (the red part indicates the DNA sequence in the DNA structure region).
As mentioned earlier, JAK kinase can phosphorylate STAT transcription factors. There are currently seven main STAT proteins, namely STA T1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6. The expression of STAT protein is widespread, and the structure contains a very conserved SH2 domain, and the C-terminal has a tyrosine residue that can be phosphorylated by JAK (Figure 3). As shown in the figure, the STAT protein can induce the formation of homodimers or heterodimers through the interaction between the SH2 domain of one molecule and the phosphorylated tyrosine residues of another molecule. In addition, because it contains a DNA binding domain, these dimers can subsequently migrate to the nucleus to further bind to the promoter sequence of the target gene (Figure 4).

Figure 4. JAK-STAT signal pathway
Different STAT proteins can be activated by specific extracellular signals: for example, STAT1 is specific to the activation of IFN; STAT3 is preferentially activated by IL6 family cytokines; STAT4 is activated by IL12, STAT6 is activated by IL4, and STAT5 is activated by IL2 and IL3 family interleukins , Hormones and hematopoietic growth factors are activated.
An important regulator in the JAK-STAT pathway is SOCS (suppressor of cytokine signalling), also known as SSI (STAT induced STAT inhibitor) or JAB (JAK-binding protein). SOCS has SH2 domain and JAK inhibition domain KIR (kinase-inhibitory region). Its SH2 domain can bind receptor phosphorylated tyrosine residues to prevent STAT protein activation; at the same time, SOCS can also pass its SOCS box (SOCS Box). ) Recruit E3 ubiquitin ligase to degrade the “reception complex” or STAT protein.
Another negative regulator in the JAK-STAT pathway is PIAS (protein inhibitor of activated STAT), which can bind to phosphorylated STAT protein to prevent its dimerization. In addition to SOCS and PIAS, SH2 domain-containing SHP phosphatase or PTPN (protein tyrosine phosphatases, non-receptor) phosphatase can reversely regulate the STAT3 signal pathway through JAK kinase dephosphorylation.
STAT transcription factors have numerous target genes. For example, STAT3 can regulate the transcriptional activation of genes such as BCL2, CCND1 (cyclin D1), VEGFA, MYC, JUN and FOS, and participate in cell survival and proliferation.
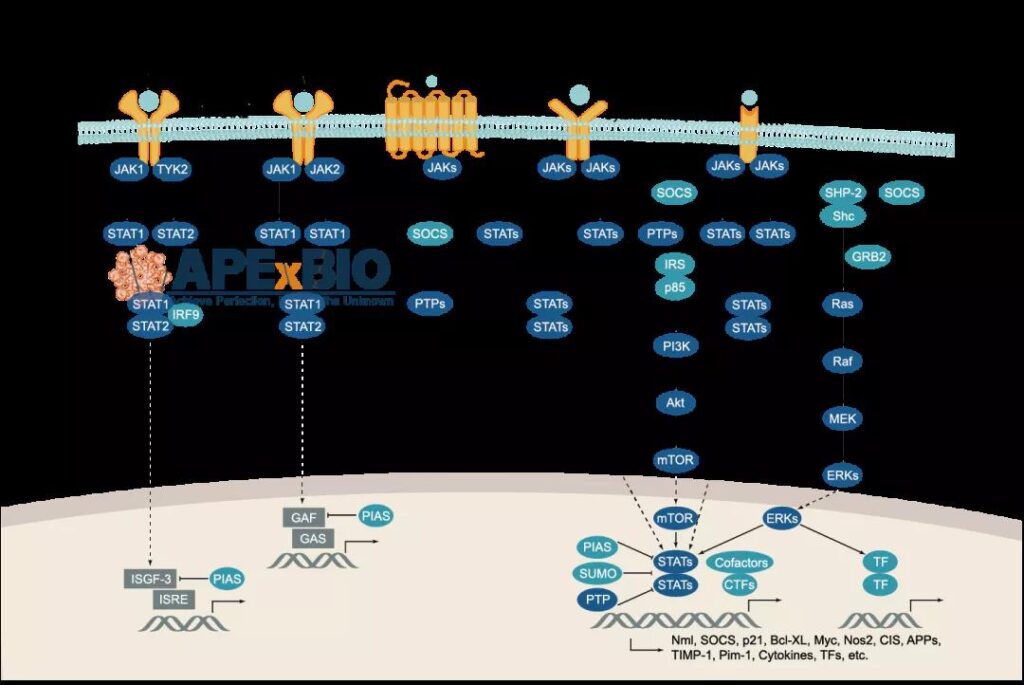
Figure 5 Multiple signal factors that activate JAK-STAT
There is an interaction between JAK-STAT pathway and many other signal pathways, which is related to the JAK signal pathway can be activated by many kinds of signals. For example, since JAK kinase has an SH2 domain, it can also recognize and bind to the phosphotyrosine residues of receptor tyrosine kinase (RTK), and is stimulated by extracellular growth factor signals to activate STAT protein. In addition, JAK protein can also participate in the activation of downstream RTK pathways, such as the MAPK (phase 3) and PI3K (phase 4) pathways.
04 JAK-STAT Pathway and Disease
1) Cytokines play a vital role in the pathogenesis of inflammatory diseases, and most cytokines complete their functional missions through the JAK-STAT signaling pathway. Therefore, the pathogenic changes of JAK-STAT pathway are related to a variety of inflammatory and immune diseases. This change can be found in almost all links of the JAK-STAT pathway, such as receptors, JAK kinases, STAT transcription factors, and SOCS.
2) Mutations in the JAK molecule itself can also lead to the occurrence of malignant tumors such as acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), ductal breast carcinoma and non-small cell lung cancer (NSCLC). For example, researchers have discovered JAK2’s JH2 domain V617F mutation in a variety of malignant diseases such as polycythemia vera, essential thrombocythemia, and idiopathic myelofibrosis. This mutation leads to the constitutive activation and susceptibility of JAK2. Body hypersensitivity.
3) Although STAT gene mutations have not been found in malignant diseases, the STAT family is over-activated in a variety of hematological tumors and solid tumors. Constitutive activation of STAT1 has been observed in acute myeloid leukemia, B-cell acute lymphoblastic leukemia and erythroid leukemia; in Hodgkin’s lymphoma, acute myeloid leukemia and certain solid tumors (prostate cancer, breast cancer, liver Over-activation of STAT3 is found in cell carcinomas, etc.; in addition, STAT5 is overexpressed in erythroid leukemia, acute myeloid leukemia, acute lymphocytic leukemia, acute megakaryocytic leukemia, and chronic myeloid leukemia.
Interestingly, although STAT is closely related to a variety of malignant diseases. However, in some cases, STAT protein has a dual role: For example, STAT1 is an apoptotic promoter in interferon signaling, which can inhibit tumors by inducing apoptosis in the late stage of tumor occurrence; IL10 can reduce inflammation-related factors by activating STAT3 Carcinogenesis process.
4) As mentioned earlier, SOCS protein can inhibit the occurrence of tumors by inhibiting the JAK pathway. However, gene inactivation caused by SOCS1 promoter methylation has been found in nearly 60% of acute myeloid leukemia, chronic myeloid leukemia and liver cancer. Similarly, SOCS3 promoter methylation is also found in liver cancer and various squamous cell carcinomas.
05 Drug research targeting JAK-STAT pathway
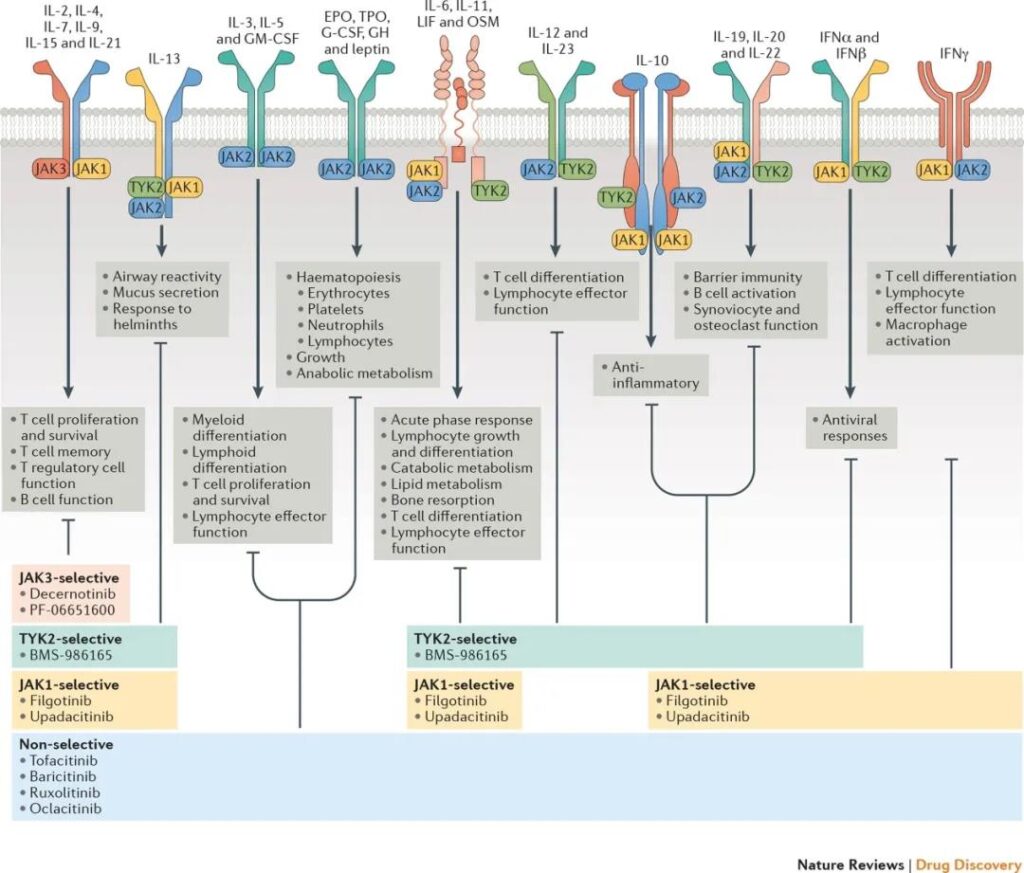
Figure 6. Drugs targeting the JAK-STAT pathway
JAK kinase is a very important drug target. JAK inhibitors developed for this target are mainly used to screen therapeutic drugs for blood system diseases, tumors, rheumatoid arthritis and psoriasis. The main targets are: JAK1, JAK3, TYK2, etc. In addition, drugs targeting STAT transcription factors, especially STAT3 and STAT5 related drugs have not been successfully developed. Currently, peptide mimics targeting the SH2 domain of STAT and STAT mRNA antisense oligonucleotides that inhibit STAT synthesis are under investigation.
(source:internet, reference only)
Disclaimer of medicaltrend.org



