Antisense oligonucleotides for long-term treatment of congenital blindness
- Did Cloud Seeding Unleash a Deluge in Dubai?
- Scientists Identify Gut Bacteria and Metabolites that Lower Diabetes Risk
- OpenAI’s Model Matches Doctors in Assessing Eye Conditions
- UK: A Smoke-Free Generation by Banning Sales to Those Born After 2009
- Deadly Mutation: A New Monkeypox Variant Emerges in the DRC
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
Antisense oligonucleotides for long-term treatment of congenital blindness
Antisense oligonucleotides for long-term treatment of congenital blindness. Congenital amaurosis (LCA) is the earliest and most serious hereditary retinopathy.
The cone cell function of both eyes is completely lost at birth or within one year after birth, leading to congenital blindness in infants and young children. It is the main disease that causes congenital blindness in children (accounting for 10%-20%).
Most of the inheritance is autosomal recessive, and different types of congenital amaurosis caused by multiple gene mutations have been found. The more common one is congenital amaurosis type 10 (LCA10) caused by CEP290 biallelic mutations.
In 2019, Artur Cideciyan and others at the Perelman School of Medicine at the University of Pennsylvania published a clinical trial paper in Nature Medicine. The research team designed an antisense oligonucleotide called sepofarsen against the CEP290 mutant gene. This short RNA molecule can be increased by The normal level of CEP290 protein in the photoreceptor cells of the eye can improve the vision of the patient. Clinical trials conducted in 10 patients have shown that repeated injection of sepofarsen into the eye every three months can continuously improve the vision of the patient.
On April 1, 2021, Artur Cideciyan and others once again published a clinical trial paper titled Durable vision improvement after a single treatment with antisense oligonucleotide sepofarsen: a case report in Nature Medicine. The research team introduced the 11th patient in detail. The patient received only one injection, and during the following 15 months of observation, the patient’s vision continued to improve.
The research team said that a single injection of antisense oligonucleotides brought such long-term vision improvement unexpectedly. This study represents a truly exciting research direction for antisense oligonucleotide therapy.

Patients with congenital amaurosis who carry a mutation in the CEP290 gene have severe visual impairment and even blindness, usually beginning in infancy. In order to treat this rare genetic disease, the research team designed and developed an antisense oligonucleotide called sepofarsen.
Sepofarsen binds to the mRNA of the mutant CEP290 gene to guide correct splicing. Then, the photoreceptor cells in the retina can again produce the correct CEP290 protein to restore vision.
Before treatment, the patient’s visual acuity (the maximum ability of the eye to distinguish the fine structures of objects) was reduced, his field of vision was narrow, and his night vision was lost. After the initial injection, the patient gave up the follow-up treatment of injection every three months because this multiple injection dose may cause cataracts.
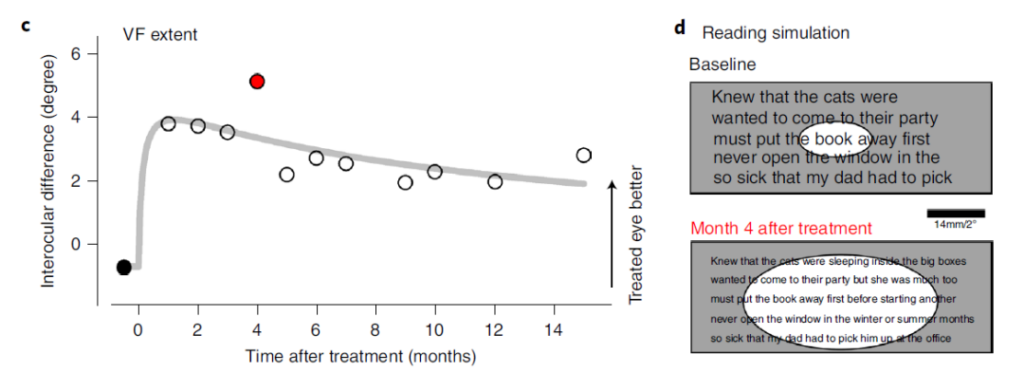
However, unexpectedly, after a single injection of Sepofarsen, the research team performed dozens of measurements on the patient’s visual function and retinal structure, and the results showed a huge improvement. The patient’s vision improved after one month. The vision peaked after two months.
Most strikingly, when the test was performed within 15 months after the first and only injection, the patient’s vision improvement still existed.
The research team said that the reason why antisense oligonucleotides can treat this rare disease is because the RNA molecules are small enough to enter the nucleus and will not be cleared quickly, so they can be retained for enough time to complete treatment effect.
The antisense oligonucleotide therapy was developed by ProQR Therapeutics, which was established in the Netherlands in 2012 to develop RNA therapies for inherited retinal diseases. The company has been listed on the Nasdaq and currently has a market value of US$328 million.

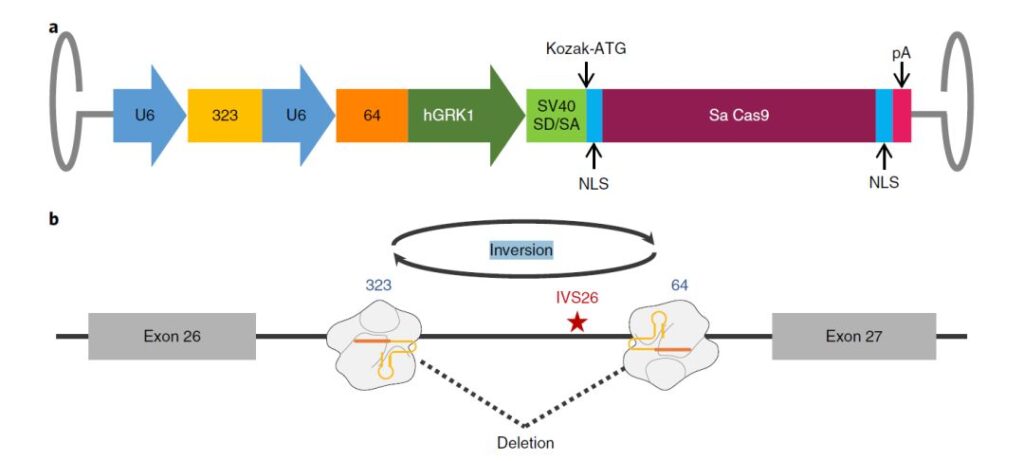
For congenital amaurosis caused by mutation of CEP290 gene, Editas Medicine founded by Feng Zhang has also carried out clinical trials. Editas Medicine uses CRISPR gene editing strategy to deliver saCas9 and CEP290-specific gRNA through subretinal injection using AAV5 vector To the photoreceptor cells, the double gRNA is used to target the upstream and downstream of the mutant intron region, and the mutant intron region is directly deleted or inverted, thereby restoring the normal expression of the CEP290 gene.
Editas Medicine was founded in 2013 and listed on the Nasdaq in 2016, becoming the first listed CRISPR gene editing technology company with a current market value of US$2.885 billion.

Artur Cideciyan of the Perelman School of Medicine at the University of Pennsylvania said that this study shows that a single injection of antisense oligonucleotides can bring long-lasting improvement in vision. This study is a treatment effect for congenital amaurosis caused by the CEP290 mutation. Improvements have brought new standards. The results of this study can also be used to compare the advantages of CRISPR gene editing and antisense oligonucleotides in the treatment of congenital amaurosis.
The research team said that it will continue to conduct related treatment research for other genetic retinal diseases that are currently incurable.
(source:internet, reference only)
Disclaimer of medicaltrend.org



