The biological clock is synchronized with the body and also with the virus…
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
The biological clock is synchronized with the body and also with the virus…
The biological clock is synchronized with the body and also with the virus… The circadian rhythm is a ubiquitous endogenous timing system (biological clock), which controls a series of biological processes in the body, including hormone secretion, metabolic circulation, and immune protection against pathogens with a cycle of approximately 24 hours.
The circadian signal transduction pathway in mammals exists in almost every cell and is mainly controlled by a series of transcription/translation feedback loops. However, this approach may also be used by viruses.
Recently, in a study published in “Nature Communications”, a research team led by the University of Oxford found that the circadian rhythm transcription factor (BMAL1) binds to the hepatitis B virus genome. This virus-host interaction promotes viral infection in cells and animal models. In other words, the circadian rhythm is still affecting the replication of hepatitis B virus. This research provides an important way for the development of new antiviral drugs.

Hepatitis B virus (HBV) is one of the major public health problems in the world. There is currently no effective treatment. According to data from the World Health Organization, more than 270 million people are infected with HBV every year, and at least 880,000 people die of liver cirrhosis and hepatocellular carcinoma.
Although there are currently interferon and nucleoside drugs for the treatment of hepatitis B, HBV can form a covalently closed circular DNA molecule (cccDNA) in the nucleus of human liver cells and integrate it into the human genome, so that its viral DNA form can exist stably. This is also the key to HBV’s ability to maintain long-term chronic infection in the body and is difficult to clear.
The biological clock is believed to be approximately 2.5 billion years old and can be traced back to cyanobacteria that released large amounts of oxygen during the Great Oxidation Event. Interestingly, many factors of human oxygen receptors are homologous to those used to sense time, and there is increasing evidence that there is an interaction between circadian rhythms and hypoxia signaling pathways.
Previous research by Jane McKeating’s team at the Nuffield School of Medicine at Oxford University has found that this ancient oxygen sensor promotes HBV replication through hypoxia-inducible factors. But has HBV evolved to use these two ancient ways (biological clock and oxygen induction) to infect the liver? This puzzle has gradually been solved.
HBV replicates in the liver, and about 20% of liver genes show a rhythmic expression pattern, which indicates that the virus has successfully evolved and persisted in this organ regulated by circadian rhythm. The circadian rhythm factors BMAL1/CLOCK and REV-ERB are the main regulators of the liver transcriptome. Recent studies have shown that BMAL1 has multiple effects of inhibiting herpes, influenza and respiratory syncytial virus while promoting the replication of human immunodeficiency virus and hepatitis C virus. This means that the circadian pathway has a dynamic central role in determining the susceptibility of the host to the virus.
In this study, the team established a circadian dHepaRG liver cell model, and proved that REV-ERB agonists inhibit the expression of HBV receptor sodium taurocholate cotransport peptide (NTCP) and HBV entry into original target cells Direct role in the. Rhythmic circulation is reduced by inhibition of agonists. In addition, the researchers demonstrated that the agonist is not toxic to HepaRG cells within the tested dose range.
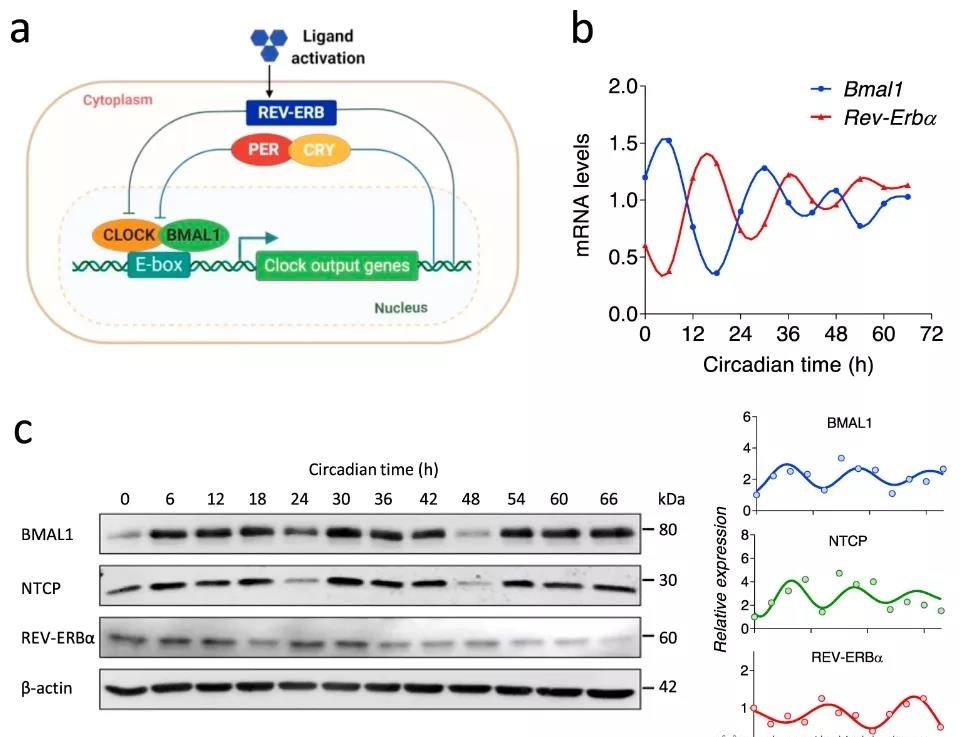
Subsequently, through experiments in human liver chimeric mice, the researchers found that the pharmacological activation of REV-ERB can inhibit HBV infection, and BMAL1 binds to the HBV genome to increase the activity of the viral promoter. The pharmacological inhibition of BMAL1 by REV-ERB ligand reduces pregenomic RNA and de novo granule secretion.
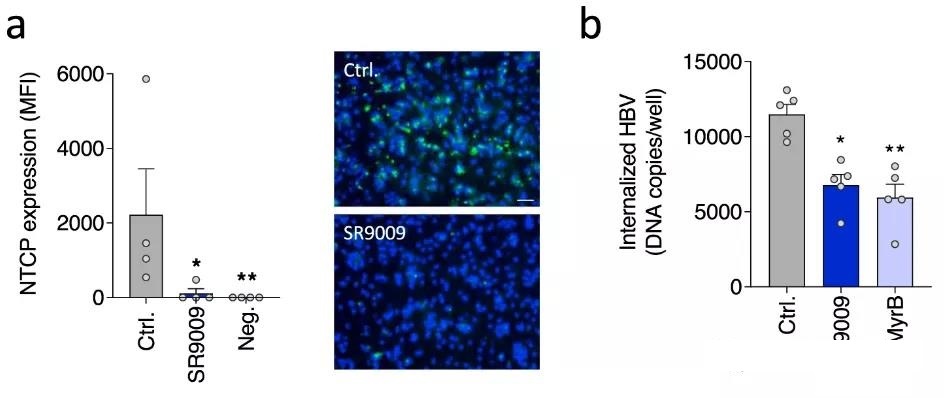
This study shows that the E-box motif in the hepatotropic DNA virus family highlights the evolutionary conservation role of BMAL1 in regulating this DNA virus family.
In short, this research provides an innovative way to deeply understand and treat the targeting of chronic HBV infection.
(source:internet, reference only)
Disclaimer of medicaltrend.org



