Linker strategy in bispecific antibody technology platform
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
- Kochi University pioneers outpatient bladder cancer treatment using semiconductor lasers
- ASPEN 2024: Nutritional Therapy Strategies for Cancer and Critically Ill Patients
- Which lung cancer patients can benefit from neoadjuvant immunotherapy?
- Heme Iron Absorption: Why Meat Matters for Women’s Iron Needs
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
Linker strategy in bispecific antibody technology platform
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Linker strategy in bispecific antibody technology platform.
The most important point of bispecific antibody design is how to improve the correct assembly of the antibody and reduce the mismatch between antibody chains.
There are already a variety of methods to prevent mismatches of bispecific antibodies. Today, this article will summarize the role of linker in preventing mismatches of bispecific antibodies.
OAscFab-IgG
The OAscFab-IgG technology was developed by the Roche team. The antibody design is mainly connected by (G4S)6GG 32 amino acid linker.
Linker starts from the N-terminus of the heavy chain of an antibody, and then connects to the C-terminus of the light chain of the antibody to promote the mismatch between the light chain of the antibody and the corresponding heavy chain. The linker is not removable.
 Figure 1: OAscFab-IgG bispecific antibody platform
Figure 1: OAscFab-IgG bispecific antibody platform
LUZ-Y
The LUZ-Y technology was invented by the Eli Lilly team. It mainly uses Acid.p1 (Ap1) and Base.p1 (Bp1) to form a parallel heterodimer, and the two peptides are added to the C-terminus of the antibody heavy chain.
After expression, the content of heterodimer is increased through the interaction between Ap1 and Bp1.
In order to remove the leucine zipper structure, the lysyl peptide chain endonuclease cleavage site (at the end of the normal antibody in Figure 2) was introduced at the C-terminus of the antibody and the N-terminus of the leucine zipper.
At the same time, to prevent the light chain error of the antibody Match, introduce the GGGS linker between the same antibody VH and CL (the black line in Figure 2).
Moreover, the design can remove the linker by introducing a furin cleavage site (the short red line in Figure 2 is the furin cleavage site).
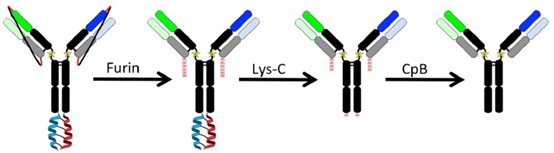 Figure 2: LUZ-Y bispecific antibody platform
Figure 2: LUZ-Y bispecific antibody platform
tcBsIgG (tethered-variableCL BsIgG)
The tcBsIgG technology platform was developed by the Genentech team. This technology mainly connects the variable region of the antibody light chain to the variable region of the antibody heavy chain through a G4S linker (Figure 3).
After expression, the variable region of the light chain of the antibody is connected to the heavy chain variable region of the antibody. The variable regions of the chain combine with each other to prevent mismatching of light chains (heavy chains mainly rely on Knob-In-Hole technology to prevent mismatching).
At the same time, the antibody’s light chain constant region CL is co-expressed with the two modified heavy chains, and CL combines with the CH1 of the antibody heavy chain by intermolecular forces and forms a stable disulfide bond.
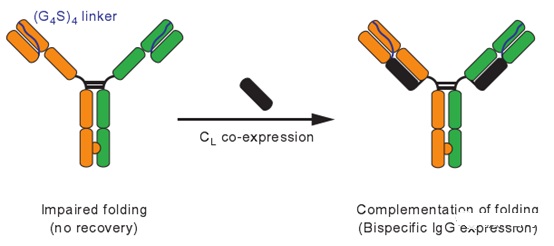 Figure 3: tcBsIgG bispecific antibody platform
Figure 3: tcBsIgG bispecific antibody platform
LegoBody
The design of LegoBody mainly uses thrombin (Thrombin), which has strong cleavage sequence specificity and high hydrolysis efficiency, and is widely used to cut tags.
In order to prevent the mismatch of the light chain of the antibody, the light chain of the antibody is connected to the corresponding heavy chain of the antibody through a linker.
The C-terminus of the light chain and the N-terminus of the heavy chain are added with sequences that can be hydrolyzed by thrombin.
After the antibody is purified, the linker is cleaved by thrombin to form a bispecific antibody without linker.
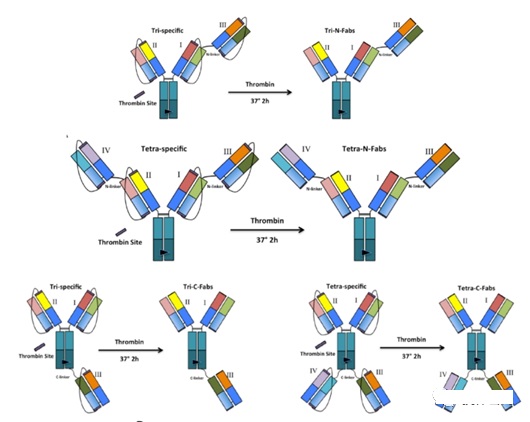
Figure 4: Multispecific antibodies constructed by LegoBody
Sum up
Among the currently known bispecific antibody platforms, these four linker platforms are used to prevent mismatches between antibody heavy and light chains.
Among them, the Linker in the LUZ-Y and LegoBody platforms can be removed by later enzyme digestion treatment.
LUZ-Y mainly uses furin and carboxypeptidase B to remove linker and residual amino acids, but it is necessary to introduce a point mutation K222A at position 222 of the hinge region of the heavy chain (to prevent non-specific cleavage by the enzyme).
LegoBody mainly uses thrombin to remove the linker, but there will be amino acid residues. The linker in the tcBsIgG and OAscFab-IgG platforms is part of the antibody and cannot be removed by later technology.
(source:internet, reference only)
Disclaimer of medicaltrend.org