Actin modulator hMENA promotes cancer cell/stromal cell cooperation
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
Actin modulator hMENA regulates GAS6-AXL axis and promotes cancer cell/stromal cell cooperation
Actin modulator hMENA promotes cancer cell/stromal cell cooperation. The dynamic interaction between tumor cells and tumor-associated fibroblasts (CAFs) is regulated by a variety of signaling pathways, which can lead to cancer progression and treatment resistance. Studies have proved that hMENA (a member of the actin regulatory protein of the Ena / VASP family) and its tissue-specific isoforms affect many intracellular signaling pathways related to cancer progression.
In 2020, Melchionna R et al. published an article titled “The actin modulator hMENA regulates GAS6-AXL axis and pro-tumor cancer/stromal cell cooperation” in EMBO reports, which reported on the hMENA / hMENAΔν6 isomer New functions in tumor-promoting CAFs and tumor-promoting cancer cell/CAF crosstalk regulated by the GAS6/AXL axis. The introduction is as follows:
LC-MS/MS proteomic analysis showed that CAFs overexpressing hMENAΔν6 secrete AXL ligand GAS6, which is beneficial to the invasiveness of pancreatic ductal adenocarcinoma (PDAC) and non-small cell lung cancer (NSCLC) cells expressing AXL.
In contrast, hMENA/hMENAΔν6 regulates the expression of AXL in tumor cells, thereby maintaining the GAS6-AXL axis, which is critical in EMT, immune escape, and drug resistance. Clinically, we found that in PDAC and NSCLC, high hMENA/GAS6/AXL gene expression characteristics are associated with poor prognosis.
We believe that hMENA promotes cancer progression through the interaction between paracrine tumors and stroma, and has profound prognostic and therapeutic significance for NSCLC and PDAC. The following will explain the specific research results of hMENA and interference with tumor progression from five aspects.
1. hMENA/hMENAΔν6 defines a tumor-promoting CAF activation state
We evaluated whether the expression of hMENA and its isoforms not only play a role in tumor cells, but also play a role in tumor-promoting CAF biology.
We isolated CAFs from the excised primary PDACs (P-CAFs) and NSCLCs (L-CAFs). The isolated CAFs showed typical spindle-shaped mesenchymal cell characteristics and lacked mutations found in the primary tumor, which was revealed by NGS analysis of 22 genes of all CAFs used for functional studies. In addition, CAF IF analysis and qRT-PCR confirmed that these cells express CAF markers (ie FAP, PDGFRB) and EPCAM negative.
The characteristics of the hMENA isoforms clearly indicate that, as expected, the epithelial hMENA11a isoforms (Figure 1A) as well as pan-cytokeratin and E-CADHERIN (Figure 1) CAFs expressed in cancer cells (EpPDAC) It was negative and was immunostained by α-SMA and Pan-hMENA monoclonal antibodies (representative case PDAC#36 in Figure 1A).
The tissue specificity of hMENA splicing confirmed by RT-PCR and WB analysis showed that CAFs expressed hMENA (88 KDa) and mesenchymal-specific hMENAΔν6 (80 KDa), but did not express hMENA11a (90 KDa).
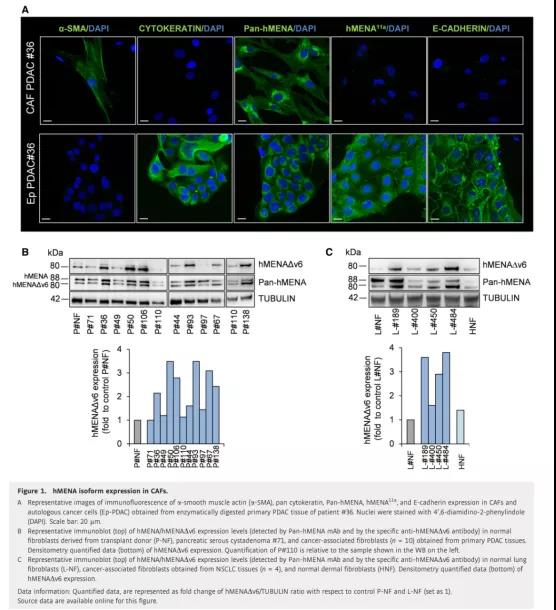
Figure 1 Expression of hMENA isoforms in CAFs
Then, we compared the expression levels of hMENA/hMENAΔν6 in normal pancreatic tissues (P-NFs) and P-CAFs fibroblasts from transplant donors. WB analysis showed that in most of the evaluated cases, the expression level of these two isoforms in P-CAF was higher than that of P-NF (Figure 1B).
L-CAF was compared with normal lung fibroblasts (L-NF) (Figure 1C) and paired “distal” fibroblasts (L-DFs) of non-“tumor” tissue at least 5 cm away from the tumor core , The results are similar. Therefore, with the exception of #97 from PDAC peritoneal metastasis (Figure 1B), hMENAΔν6 was expressed in all non-permanent CAF cultures we tested (Figures 1B and C), despite the heterogeneity of expression levels.
In addition, we were able to isolate fibroblasts from pancreatic serous cystadenoma #71, showing very low hMENA/hMENAΔν6 expression (Figure 1B).
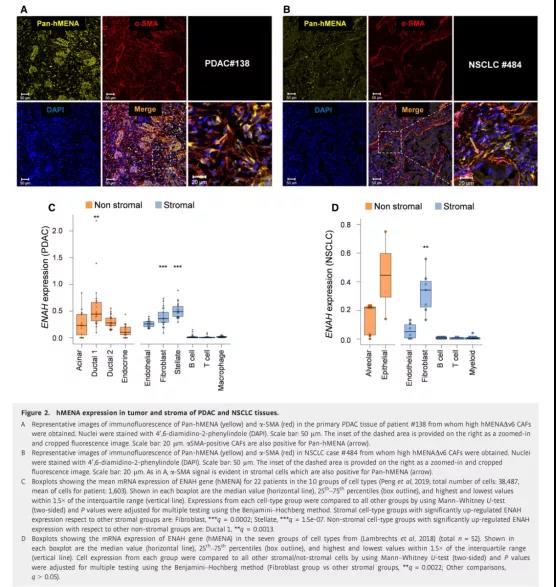
Figure 2 Expression of hMENA in tumor cells and stromal cells of PDAC and NSCLC tissues
The IHC analysis of primary NSCLC tissues also confirmed the heterogeneous expression of hMENA in the matrix. In order to confirm the presence of CAF in primary tumor tissues overexpressing hMENA/hMENAΔν6, we performed confocal analysis on NSCLC and PDAC tissues co-stained with Pan-hMENA and α-SMA antibody. The results showed that Pan-hMENA modified α-SMA positive The stromal cells, as expected, were also modified with α-SMA-negative tumor cells (Figure 2A and B).
In addition, in the PDAC single-cell RNA-Seq data, we demonstrated that compared with other stromal cell types, hMENA is expressed at higher levels in fibroblasts and stellate cells, but not in immune cells (Figure 2C). However, compared with other tumor cells, the expression level of hEMNA in Ductal 1 cells is higher. Similarly, single-cell RNA-Seq of the lung tumor microenvironment identified different stromal cell subtypes. Through this analysis, the result we collected is that compared with other types of stromal cells, the expression level of hEMNA (ENAH) in fibroblasts is higher (despite the heterogeneity between clusters) (Figure 2D).
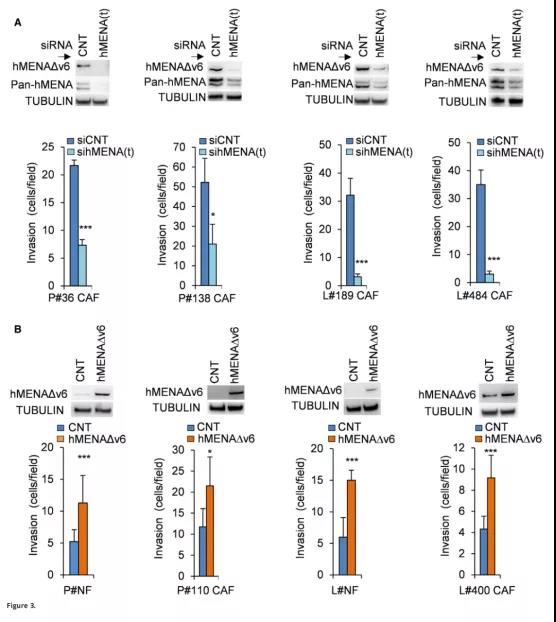

Figure 3 hMENA/hMENAΔν6 regulates the functional activity of tumor-promoting CAF
In order to further explain the functional significance of hMENA/hMENAΔν6, we conducted functional experiments in multiple P-CAF and L-CAF and P-NF and L-NF to analyze whether the expression level of hMENA/hMENAΔν6 is related to CAF activity. We found that, relative to P-NF and L-NFs, the high expression of hMENA/hMENAΔν6 in CAFs is related to collagen gel contraction capacity (a measure of matrix remodeling capacity) and the ability to secrete and activate MMP-2. Compared with normal primary fibroblast NFs, the increase in FAP expression in these cells confirmed the activation phenotype of CAFs.
When we use three different siRNAs (sihMENA(t)) in CAFs with high expression of hMENAΔν6, (PCAF#36; P-CAF#138; L-CAF#189; L-CAF#484, Figure 1B and C) This hMENA-related CAF function (Figure 3) is obvious when all hMENA isoforms are silenced. Through the Matrigel transwell invasion experiment (Figure 3A), it was found that SihMENA(t) reduced the invasion ability of CAFs and activated MMP-2 in L-CAFs and P-CAFs. In addition, we observed that compared with the control group, the ability of CAFs to shrink the collagen gel in hMENA/hMENAΔν6 silenced CAFs was significantly reduced.
When we overexpress hMENAΔν6 (P-CAF#110 and L-CAF#400, Figures 1B and C) in P-NFs and L-NFs and CAFs with low expression of hMENAΔν6, we found that non-tumor fibroblasts and low expression The CAFs of hMENAΔν6 increased their functional activity (Figure 3B).
In summary, these data indicate for the first time the role of hMENA/hMENAΔν6 as a marker for tumor-promoting CAF activation.
2. hMENA plays a vital role in the synergy between tumor cells and CAFs
It is well known that CAFs promote tumor progression and invasion through the activation of paracrine signals in various cancers.
In order to determine whether hMENA/hMENAΔν6 expression affects the paracrine pro-invasion pathway, we collected conditioned media (CM) from P-NFs and P-CAFs. According to the expression of hMENAΔν6 in WB analysis, it was divided into P-CAFhigh (hMENAΔν6 expression was higher than 2 times the average expression of NFs) and P-CAFlow (hMENAΔν6 expression was lower than 2 times; Figure 1B).
We evaluated the effect of CM on the invasiveness of PANC-1 cells and found that when PANC-1 was treated with P-CAF-CM for 48 hours, the increase in cancer cell invasion was related to the high expression of hMENAΔν6. Indeed, as shown in Figure 4A, compared with CM derived from P-CAFlow and/or NFs, CM derived from P-CAFshigh has a higher pro-invasive effect (Figure 4A), indicating that CAF-CM increases cancer cell invasion.
The ability is related to the expression of hMENA/hMENAΔν6. It was agreed that when CAFs silenced hMENA/hMENAΔν6, their CM could not induce PANC-1 and KP4PDAC cell invasion (Figure 4B). These data were confirmed in H1975 (Figure 4C and D) and A549 NSCLC cells. In addition, CM that silenced P-CAFs also showed a reduced ability to induce tumor cell growth in vitro.
These results indicate that the overexpression of hMENA/hMENAΔν6 determines the subgroup of CAFs with pro-tumor function, which can regulate the growth and invasion of tumor cells by regulating paracrine factors.
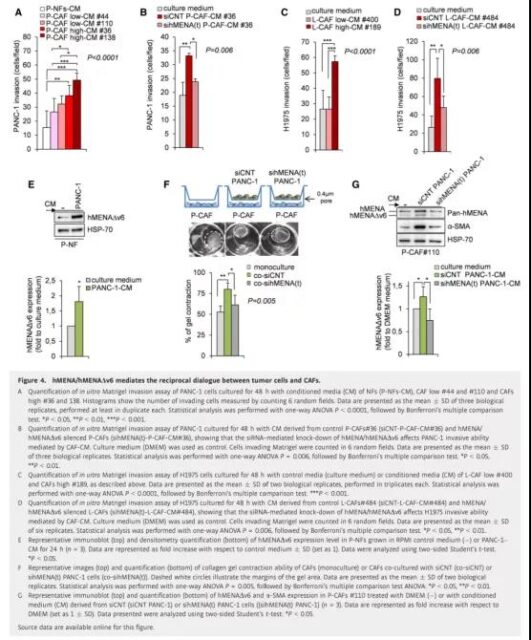
Figure 4 hMENA/hMENAΔν6 mediates the mutual dialogue between tumor cells and CAFs
Based on the correlation of two-way communication between cancer cells and CAFs, we tested whether hMENA-mediated CAF activation is maintained by the overexpression of hMENA in tumor cells. We first treated P-NFs with CM from PANC-1 cells (highly expressing hMENA/hMENAΔν6 isoform) for 24 hours, and then evaluated the expression of hMENAΔν6 in P-NFs and P-CAF#110. We found that tumor cell-derived secretory factors significantly up-regulated hMENAΔν6, suggesting that the tumor-derived secretory group can induce hMENAΔν6 overexpression in NFs (Figure 4E). This situation also occurs in CAFs with low hMENAΔν6 levels (P-CAF#110) (Figure 4G) and high hMENAΔν6 levels (L-CAF#484).
In order to understand whether hMENA/hMENAΔν6 in tumor cells supports CAF activation, CAF was transwelled with control PANC-1 cells (siCNT PANC-1) and/or with hMENA/hMENAΔν6 silenced PANC-1 cells (sihMENA(t) PANC-1) Single culture or indirect co-culture. After 48 hours, we observed that the co-cultivation of P-CAFs with control tumor cells (siCNT PANC-1) increased the gel contraction ability of CAF compared with the single-cultured P-CAFs (Figure 4F). Relatedly, the silencing of hMENA/hMENAΔν6 expression in PANC-1 inhibited the activation of CAF, which can be indicated by the decrease in the gel contraction ability of CAFs induced by CM derived from PANC-1 silenced cells (Figure 4F). We also found that CM from PANC-1 cells (control) induced the expression of hMENAΔν6 and α-SMA in CAFs (#110 low hMENAΔν6). When we silenced hMENA/hMENAΔν6 (sihMENA(t) PANC-1), this The effect is eliminated (Figure 4G). These data indicate that hMENA regulates the interaction between CAF and tumor cells through paracrine factors.
3. The expression of hMENA/hMENAΔν6 in CAFs regulates cancer cell invasion through GAS6
In order to identify secreted proteins that may explain the difference in tumor-promoting functions between CAFhigh and CAFlow, we performed LC-MS/MS proteomics analysis on CMs from P-CAFhigh, P-CAFlow and P-NFs.
We identified 142, 321 and 387 CM-NFs, CM-CAFlow and CM-CAFhigh proteins, respectively.
Among the proteins identified in all CM samples, there are a total of 102 proteins. Compared with CM-NFs, 25 proteins in CM-CAFhigh are up-regulated, and compared with CM-CAFlow, 16 proteins in CM-CAFhigh are up-regulated. . It is worth noting that 10 of these proteins (ie SYNC, CACNA1A, EPHA3, MUC16, SIK3, CDH13, GAS6, MYH9, ZBBF2 and CCD37) are the only ones secreted by CM-CAF high and are defined as hMENAΔν6 related proteins (Figure 5A).
Among the 10 proteins identified, considering the main role of its receptor AXL in the resistance mechanism, we focused on the growth arrest-specific protein 6 (GAS6). The resistance mechanism is mainly mediated by EMT, including resistance to ICB. Resistance.
We verified by ELISA and qRT-PCR (Figure 5B and C) that P-CAFshigh highly secretes GAS6 compared with P-CAFslow and P-NFs. The difference is that we found that PANC-1 cells express low levels of GAS6 (Figure 5D), which is consistent with the previous data, indicating that GAS6, as a matrix-derived factor, participates in the promotion of tumor paracrine-mediated communication.
We also found that compared with L-DFs, the expression of GAS6 in L-CAFs was significantly increased (P=0.0208) (Figure 5E), which is consistent with our LC-MS/MS proteomic analysis in PDAC fibroblasts .
Then we analyzed the mRNA expression of P-CAFs and L-CAFs that silenced hMENA/hMENAΔν6 to explore the role of hMENA/hMENAΔν6 in the regulation of GAS6. As shown in Figure 5F and G, hMENA/hMENAΔν6 silencing significantly reduced the mRNA expression of GAS6 in P-CAFs and L-CAFs. On the other hand, GAS6 silencing did not affect the expression of hMENA/hMENAΔν6.
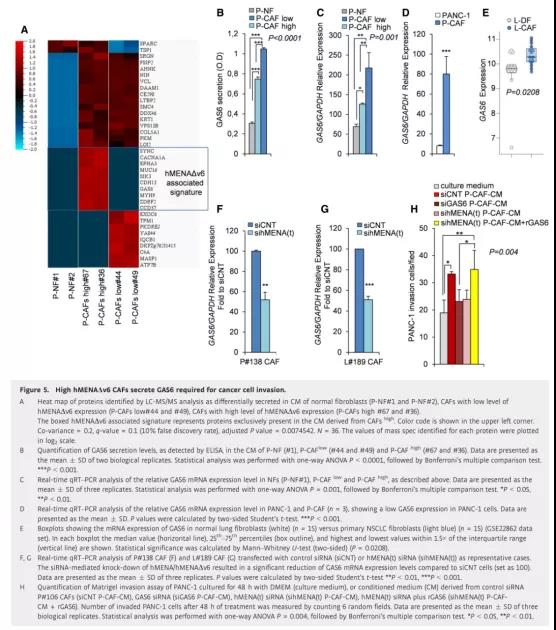
Figure 5 High hMENAΔν6 CAFs can secrete GAS6 required for cancer cell invasion
Furthermore, we asked whether the role of hMENA/hMENAΔν6 in CAF-driven tumor cell invasion depends on the ability of hMENA to regulate GAS6 secretion. To this end, we treated PANC-1 with CM derived from GAS6 expression silenced P-CAFs for 48 hours, and we observed that GAS6 silencing in CAFs reduced tumor cell invasion (Figure 5H), which had a silencing effect on hMENA/hMENAΔν6 in CAFs. Same (Figure 4B and D). This pro-invasive GAS6 derived from CAF was also obtained in L-CAFs.
Importantly, adding recombinant GAS6 (rGAS6) to hMENA(t)-silenced CAFs can rescue PANC-1’s cancer cell invasiveness (Figure 5H). These data indicate that hMENA/hMENAΔν6 regulates the expression and secretion of GAS6 in CAFs, thereby regulating CAF-mediated cancer cell invasion.
4. hMENA/hMENAΔν6 regulates the expression of AXL in tumor cells and maintains the paracrine GAS6-AXL-mediated tumor cell/CAF pro-invasion synergy
GAS6-dependent activation of the receptor tyrosine kinase AXL has been shown to increase invasive function. We recently reported that hMENA/hMENAΔν6 plays a key role in the invasive activity induced by ET-1/β-arr1 and the invasiveness of ovarian cancer.
In order to clarify the role of hMENA in the synergy of tumor and CAF cell invasion, we first tested whether hMENA regulates the expression of AXL receptor in tumor cells. We found that silencing hMENA (sihMENA(t)) reduced the levels of AXL protein in PANC-1 (Figure 6A) and KP4 PDAC and A549 (Figure 6A) H1650 and H1975 NSCLC. The mRNA levels of PANC-1 and A549 cells also showed this reduction (Figure 6B).
In order to evaluate the effect of hMENA silencing on AXL gene transcription, we measured total AXL mRNA and total RNA from total RNA isolated from siCNT and sihMENA(t)PANC-1 and A549 cell lines 72 hours after siRNA transfection by qRT-PCR. AXL precursor mRNA.
As shown in Figure 6C, the precursor mRNA and mature mRNA AXL levels in hMENA-silenced cells decreased, indicating that hMENA regulates AXL at the transcriptional level (Figure 6C). The reduced sensitivity of hMENA(t)-silenced cancer cells to the AXL expression-dependent BGB324 kinase inhibitor (R428) further confirms that hMENA(t) silence induces the down-regulation of AXL. However, in a group of cancer cell lines, BGB324 did not affect the expression of hMENA.
In order to study the role of hMENA in the ligand-dependent AXL signaling pathway, we treated PANC-1 cells with rGAS6. We found that hMENA silencing inhibited GAS6-mediated phosphorylation of AXL and AKT (Figure 6D) and passed the Matrigel transwell invasion experiment. It was detected that the invasion of GAS6 by cancer cells was reduced (Figure 6E). Therefore, these data have also been validated in the NSCLC cell line H1975. It is worth noting that, according to the pro-invasive effect of CAF-derived GAS6 on PANC-1 (Figure 5H), when treated with CAF-CM, hMENA-silenced tumor cells are less aggressive (Figure 6F). These data indicate that hMENA plays an important pro-invasive role in the communication between cancer cells and CAFs by regulating the GAS6/AXL paracrine axis.
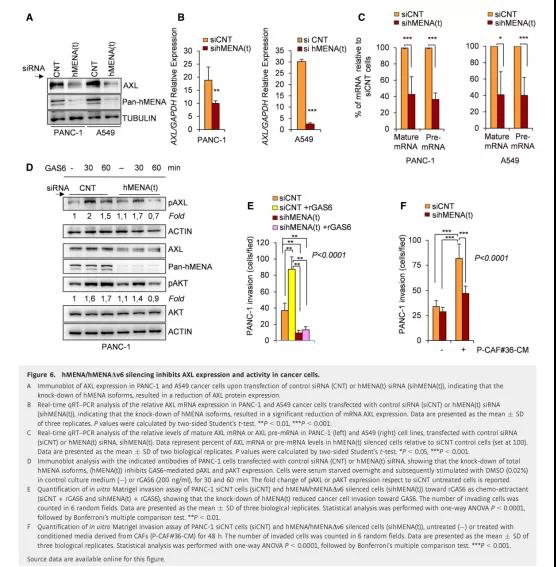
Figure 6 hMENA/hMENAΔν6 silencing inhibits the expression and activity of AXL in cancer cells
5. The combined expression of hMENA, AXL and GAS6 are related gene markers of poor prognosis in patients with pancreatic adenocarcinoma and lung squamous cell carcinoma
In order to clarify the relevance of our experimental data in clinical practice, we studied the combined expression levels of ENAH (hMENA), AXL and GAS6 mRNA in pancreatic cancer (PDAC) patients (n=172) and lung squamous cell carcinoma (LUSC) patients ( Prognostic value in patients with n=501) and lung adenocarcinoma (LUAD) (n=516).
Interestingly, the expression characteristics of 3-genes (ENAH, AXL, and GAS6) are associated with worse overall survival (OS) prognosis of PDAC, and patients are stratified according to the characteristic expression (Figure 7A, left). On the contrary, no 1 Gene characteristics (ENAH) (right side of Fig. 7A) or expression characteristics of 2 genes (AXL and GAS6) (middle part of Fig. 7A) are correlated with prognosis. It is worth noting that the elevated expression of 3 genes, rather than the characteristic expressions of 1 or 2 genes, is also associated with shorter disease-specific survival (DSS), emphasizing the clinical relevance of these three genes in PDAC, It also shows that the combined expression of ENAH, AXL and GAS6 endows the prognostic value of this gene feature.
In LUSC patients (OS 80 months), similar prognostic correlation was found with 3 gene expression characteristics (Figure 7B, left), but not 1 or 2 gene expression characteristics (Figure 7B, right).
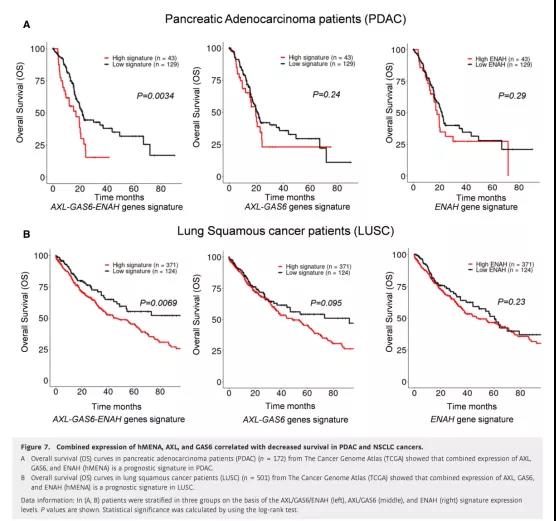
Figure 7 The combined expression of hMENA, AXL, and GAS6 is associated with decreased survival of PDAC and NSCLC
In summary, these results confirm the expression characteristics of 3-genes (ENAH, AXL, and GAS6) as prognostic indicators of aggressive disease in PDAC and LUSC patients, and strengthen the clinical relevance of hMENA expression pattern analysis in tumor cells and CAFs.
Discusion
We propose that inhibiting the actin cytoskeleton regulatory protein hMENA may interrupt the communication between cancer cells and CAFs, thereby inhibiting tumor invasiveness by regulating the GAS6/AXL axis, and affecting the prognosis of patients with PDAC and NSCLC.
Our previous research data indicate that hMENA/hMENAΔν6 is involved in the maturation of aggressive cells and mediates the invasion of cancer cells. Here we demonstrate that hMENA regulates the expression of RTK AXL, which is involved in the formation of aggressive cells. Correspondingly, we found that hMENA/hMENAΔν6 regulates the expression and secretion of AXL ligand GAS6 in CAFs, indicating that hMENA plays a key role in tumor invasiveness mediated by the synergy between cancer cells and CAFs.
We found that the expression level of hMENA/hMENAΔν6 is very low in normal fibroblasts, while it is highly expressed in activated CAFs (Figure 1B and C, and Figure 3). In primary PDAC and NSCLC tissues, hMENA/hMENAΔν6 is expressed in CAF (Figure 2 A and B). The data in the comprehensive catalog of stromal cells described at single-cell resolution proves this result. hMENA is only expressed in CAFs. Not expressed in immune cells (Figure 2C and D).
Through the analysis of the “Navib Data Set”, it was found that the expression of α-SMA and FAP in the CAF subgroups with high hMENA expression increased compared with the CAFs with low hMENA expression. Considering the main role of hMENA in the dynamic regulation of actin, we believe that hMENA does not recognize specific CAF subtypes, but reflects the pro-invasive CAF state.
In fact, we confirmed that the silencing of hMENA/hMENAΔν6 reduces the function of tumor-promoting CAF. In contrast, the overexpression of the hMENAΔν6 isoform in normal fibroblasts leads to CAF activation (Figure 3B), which in turn leads to cancer cell invasion (Figure 4A–D).
We assume that the secretion group of CAFs with high expression of hMENA/hMENAΔν6 is mainly pro-invasion factors. Through LC-MS/MS proteomics analysis, a series of proteins defining hMENAΔν6 related characteristics (ie SYNC, CACNA1A, EPHA3, MUC16, SIK3, CDH13, GAS6, MyH9, ZBBF2 and CCD37) (Figure 5A).
Studies have shown that GAS6 is a key factor involved in the paracrine stromal-tumor cell interaction, and the GAS6-AXL axis is involved in the mutual signal transmission between PDAC and stromal cells induced by the KRASG12D mutation.
Therefore, we deeply analyzed whether hMENA is actively involved in the regulation of the GAS6-AXL pathway. Our interest also depends on the relevance of this axis in drug resistance, mainly through the combination of AXL targeting compounds and immune checkpoint inhibitors through EMT, immunosuppression and recent indications.
Our data showed that silencing hMENA/hMENAΔν6 in CAFs reduced the expression and secretion of GAS6, thereby inhibiting CAF-induced cancer cell invasiveness (Figure 5B, C, F, G, H), which is consistent with a recent study It is consistent that CAF-derived GAS6 can activate AXL and promote tumor cell migration.
Based on biological and clinical significance, we report that hMENA/hMENAΔν6 plays a dual role in the regulation of the GAS6/AXL axis. In fact, the expression of hMENA not only promoted the secretion of GAS6 in CAFs, but also regulated the expression of AXL in tumor cells and GAS6-mediated AXL activation (Figure 6A-E).
We have previously demonstrated that hMENAΔν6 regulates the expression of vimentin at the RNA and protein levels, and EMT-induced vimentin is associated with the up-regulation of AXL expression.
Although the exact role of hMENA in the regulation of AXL expression remains to be fully elucidated, we have found that hMENA silencing reduces the expression levels of total mRNA and AXL precursor mRNA, indicating that hMENA contributes to AXL transcription (Figure 6C).
Functionally, the down-regulation of hMENA/hMENAΔν6 expression in tumor cells inhibited GAS6-induced cancer cell invasiveness, indicating that the expression of hMENA in tumor cells and CAFs may enhance the paracrine GAS6-AXL axis.
Clinically, in a large number of clinical samples of PDAC and NSCLC, the integrated high expression level of ENAH/AXL/GAS6 was positively correlated with poor prognosis (Figure 7A and B), providing a new predictor for the progression of PDAC and NSCLC.
From a therapeutic point of view, hMENA can regulate the activation of tumor-promoting CAF, and their interaction with tumor cells makes the protein and its tissue-specific splicing isoforms become attractive targets for cancer treatment.
Expert Comments:
The occurrence and progression of cancer has always been a hot topic in medical research, and the development, treatment and prognosis of cancer are closely related to the characteristics of cancer cells. It is well known that the interaction between cancer cells and non-cancerous organ-specific cells can affect the development of tumors.
Many studies have pointed out that cancer cell/CAF interaction and the relative autocrine and paracrine signal transduction that they activate have an important influence on tumor progression. However, there are some factors that can drive malignant cell programs and CAF cells to reprogram each other, which has important therapeutic significance.
From the perspective of cytoskeleton dynamics, a series of substances such as the actin regulator hMENA between cells or within cells may affect the communication between cells, further affect the aggressiveness of tumors, and then affect the prognosis of tumors. .
From a diagnostic point of view, by detecting the expression of some proteins or gene fragments, the prognosis of a certain tumor can be more accurately predicted, and from a therapeutic point of view, by intervening in the expression of these proteins or applying the protein to the tissues Specific splicing isoforms, thereby intervening the interaction between the protein and tumor cells, to achieve the purpose of treatment.
This provides us with new ideas for cancer research and treatment.
(source:internet, reference only)
Disclaimer of medicaltrend.org



