Immune globulin and complement levels correlate with COVID-19 patients
- Did Cloud Seeding Unleash a Deluge in Dubai?
- Scientists Identify Gut Bacteria and Metabolites that Lower Diabetes Risk
- OpenAI’s Model Matches Doctors in Assessing Eye Conditions
- UK: A Smoke-Free Generation by Banning Sales to Those Born After 2009
- Deadly Mutation: A New Monkeypox Variant Emerges in the DRC
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
Immune globulin and complement levels correlate with the severity of COVID-19 patients
Immune globulin and complement levels correlate with COVID-19 patients. Researchers speculate that the serum C4 and IgG concentrations of severe COVID-19 patients decrease, and a large number of antigen-antibody complexes form a pathway to activate the classical antibody complement system.
Literature information:
A Marcos-Jimenez, S Sanchez-Alonso, A Alcaraz-Serna, et al. Deregulated cellular circuits driving immunoglobulins and complement consumption associate with the severity of COVID-19 patients. European Journal of Immunology, 2020, 06.
Research background
Humoral immunity plays a key role in the initial control of virus infection and cell-to-cell transmission. It is mediated by the complement system and immunoglobulin (Ig). The components of different pathogens, such as the related molecules and N protein of the COVID-19 virus, together with the C-reactive protein (CRP) in the plasma may activate the selective or lectin pathway of the complement cascade in the early stages of infection.
In addition, early IgM isotype antibodies effectively trigger the classical complement cascade. The secreted IgA antibody neutralizes viruses in the mucosa of the respiratory and gastrointestinal tract.
Finally, in a more advanced stage of the disease, virus-specific IgG antibodies opsonize virus particles. This may form immune complexes involved in activating the classical complement pathway. In addition, the opsonized virus particles can adhere to the Fc receptors of phagocytes and NK cells. The latter are the main innate immune mediators in the process of antiviral response. They kill virus-infected cells through different mechanisms.
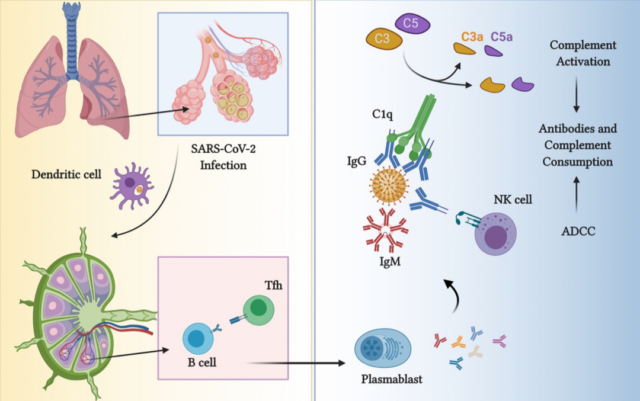
However, an excessive humoral immune response can damage host tissues. Complement-mediated tissue damage is caused by a strong inflammatory circuit triggered by C3a- and C5a-mediated recruitment and activation of neutrophils, monocytes, macrophages, lymphocytes and platelets. Phagocytes in turn produce reactive oxygen species (ROS) and proteases, and release neutrophil extracellular trap sterilization networks (NETs), which can exacerbate tissue damage.
Some studies on COVID-19 virus infection attempt to clarify the phenotypic characteristics of immune cell subsets related to disease severity or prognosis prediction. However, most of these studies were conducted under a limited number of patients and clinical conditions, and the parameters and severity of the disease were not recorded in all cases. Although specific antibodies and complement activation are thought to regulate some of the most serious complications of coronavirus (including COVID-19 virus) infection, the role of humoral immune effect mechanisms in the pathogenesis of COVID-19 virus-related ARDS is clearly needed Conclusive evidence.
To explain this problem, we analyzed the immune cell populations and soluble media of 276 COVID-19 patients. These patients were patients with mild to severe symptoms that appeared in a center during the peak of the pandemic in Madrid, Spain.
B-cells and plasmablasts in peripheral blood of patients with COVID-19 increase
According to the severity of clinical symptoms, 276 cases of COVID-19 virus infection were classified as mild, moderate, and severe. The average duration of symptoms before admission was (7.36±5.2) days, and the median age was 63 (53.25-75), of which 163 (59.05%) were male.
Preliminary analysis was carried out according to the admission plan of COVID-19 patients, which included the use of multi-parameter flow cytometry to quantify the main lymphocyte subsets in peripheral blood, including T, B, NK lymphocytes and plasmablasts.
The ratio of T lymphocytes (CD4+ or CD8+) to NK cells is similar in all patients and healthy volunteers, but the ratio of CD8+ T cells decreases in critically ill patients.
The percentage of B cells in COVID-19 patients is higher, and it increases with the severity of the disease, from an average of 9.15% in healthy volunteers to an average of 20.49% in severely ill patients.
At the same time, the relative and absolute values of plasmablasts in patients were significantly increased (average 1.51% vs 17.98%; 2.83 /L vs 27.37 /L), regardless of severity.
The increase in the number of plasmablasts indicates the redistribution of the main maturation stages of the B cell line, including naive, transitional, unswitched memory, IgM-only memory, and category-switched memory B cells, which were identified in our initial cohort of 84 patient subgroups come out.
In COVID-19 patients, only the proportion of IgM memory B cells increased, and the proportion of non-switched memory cells decreased. This redistribution is more obvious in mild patients, and gradually decreases in moderate and severe patients. There was no statistically significant difference between patients of different severity.
Patients have increased follicular helper T cells in peripheral blood
In view of the role of Tfh in B cell maturation and activation, we assessed whether the circulating Tfh (cTfh) in the peripheral blood of COVID-19 patients increases with the increase in the number of plasmablasts.
As a result, it was observed that the proportion of cTfh increased significantly with the severity of COVID-19 individuals, from a median of 0.51% in healthy volunteers to 1.7% in severely ill patients. The important thing is that although the total number of CD4+ T cells has fallen sharply, their absolute numbers have remained stable.
In order to further identify this population, we evaluated the surface expression of CCR7 chemokine receptor, which is related to the reduction of B cell activation ability by Tfh. We found that the expression of CCR7 in cTfh cells of patients with moderate to severe disease increased significantly.
Finally, we found that the ratio of cTfh is directly related to the total number of B cells (r 0.33; p = 0.0090), classification conversion B cells (r 0.26, p = 0.0433), and plasma blasts (r 0.33, p = 0.0091).
The severity of COVID-19 patients is related to serum immunoglobulin and complement levels
The high number of plasmablasts prompted us to study possible changes in immunoglobulin concentration. At the time of admission, the serum IgG, IgA, or IgM concentrations of patients with COVID-19 were comparable to those of healthy volunteers. On the other hand, when analyzing patients of different severity levels, we observed that compared with healthy volunteers, severely ill patients had similar levels of IgA and IgM, but reduced IgG concentration (see Figure 3).
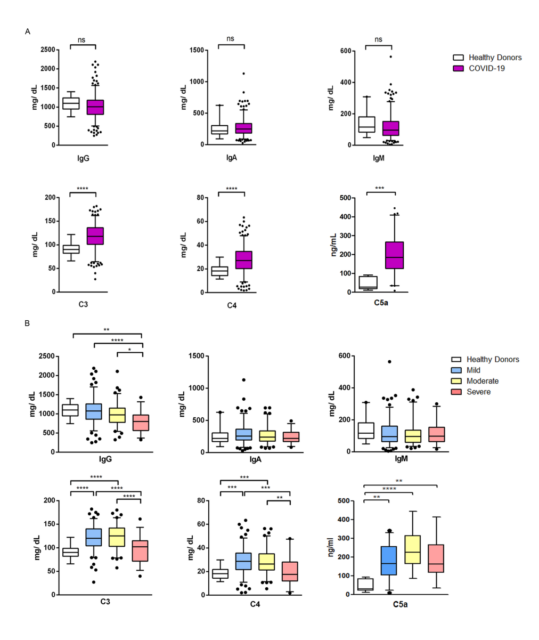
(Figure 3. Immune globulin and complement levels in COVID-19 patients)
Note: IgG, IgA, IgM, C3, C4 are detected by the turbidimetric method, and C5a is detected by the Elisa method
In addition, IgG (0.37; p=0.0007) and IgA (0.23; (p=0.0444), the serum concentration of COVID-19 patients and the absolute number of peripheral plasma blast cells, as well as the level of IgM and the total number of IgM memory B cells in peripheral blood ( There is a direct correlation between 0.36; p=0.0040). On the contrary, IgG (-0.31; p=0.0147) and IgA (-0.38; p=0.0021) are negatively correlated with the proportion of non-switched memory B cells.
COVID-19 patients are characterized by elevated plasma IL-6 and acute-phase reactant concentrations, which suggests that the concentrations of complement C3 and C4 (considered acute-phase reactants) may also increase. Therefore, we detected the C3 and C4 levels in the serum of these patients, and the increase in the levels of these two complement proteins can be detected (Figure 3A).
Therefore, in order to study whether there is complement activation in COVID-19 patients, we quantified the activating peptide C5a of complement component C5, which showed elevated plasma levels in most patients.
However, when considering the different severity levels, we found that the C3 and C4 levels of patients with mild to moderate disease increased, while the C3 and C4 levels of patients with severe disease returned to levels similar to those of healthy volunteers. Therefore, compared with other inflammatory parameters such as LDH, ferritin or CRP, C3 and C4 values decrease as the disease worsens. It is worth noting that, unlike C3 and C4, the level of C5a in plasma is still elevated regardless of the severity.
Next, we tested whether the reduction of these humoral immune components is related to the severity of the disease in critically ill patients, or more precisely reflects the increase in long-term consumption. Then, we considered taking a hospital stay longer than 15 days as a reading of the severity of the disease, and collected the blood of 37 COVID-19 patients who were still in the hospital 10 days after admission and compared it with the initial blood test.
Through this method, we observed that all patients had similar concentrations of IgG, IgA and IgM antibodies, IgG subclasses IgG1, IgG3 and IgG4, and complement proteins C3, C4 and C5a on admission. However, for patients who were hospitalized for more than 15 days, the concentration of IgG in the serum decreased significantly after 10 days, while the levels of IgA and IgM remained unchanged during this period.
Interestingly, although the IgG1 and IgG3 levels of long-term hospitalized patients significantly doubled 10 days after admission compared to short-term hospitalized patients, the decline in IgG4 concentrations in the two groups of patients was similar. In order to determine whether these changes are related to a specific anti-SARS-CoV-2 response, we quantified anti-SARS-CoV-2 RBD and N protein IgG, which can be detected in high titers on day 10 in all cases Of antibodies. In contrast, the levels of anti-CMV IgG decreased in the two groups after 10 days of hospitalization.
Regarding complement, the C3 levels of the two groups of patients were stable over time. In patients whose severity ultimately required longer hospitalization, the C4 concentration dropped significantly after 10 days (Figure 4A). However, in these patients, C5a levels persisted at this time (Figure 4A). In contrast, in patients who were hospitalized for less than 15 days, the activation product C5a increased, but there was no parallel decrease in C4 (Figure 4A).
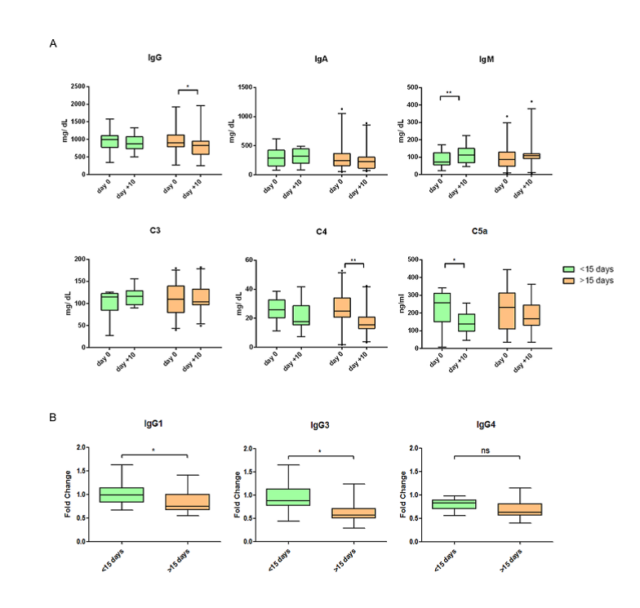
(Figure 4. The relationship between the humoral immune response and the severity of COVID-19 patients)
We speculate that the serum C4 and IgG concentrations of severe COVID-19 patients decrease, and a large number of antigen-antibody complexes form a pathway to activate the classical antibody complement system.
Although the immune system is necessary to protect against COVID-19 virus infection, if its response is excessive or lasts for a period of time, such as in COVID-19 patients, there may be persistent inflammation and hypercoagulability.
Therefore, it is necessary to provide more insights on individual characteristics and the special dynamics of the host’s response to SARS-CoV-2 to further understand the pathogenesis of COVID-19 and determine the appropriate treatment and treatment timing.
This knowledge has special therapeutic significance because it is the basis for clinical trials of different immunomodulators currently available (including intravenous immunoglobulin, hyperimmune plasma or complement inhibitors, etc.).
The dynamic monitoring of immune indicators in patients with severe infection can provide a reference for adjusting the treatment plan of patients. The national competition biological immunoscattering turbidimetric method detects 5 immune items (IgG, IgA, IgM, C3, C4), and provides experimental solutions for the monitoring of humoral immune response.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



