New drug for obesity: Imcivree is recommended and approved by EU CHMP
- Did Cloud Seeding Unleash a Deluge in Dubai?
- Scientists Identify Gut Bacteria and Metabolites that Lower Diabetes Risk
- OpenAI’s Model Matches Doctors in Assessing Eye Conditions
- UK: A Smoke-Free Generation by Banning Sales to Those Born After 2009
- Deadly Mutation: A New Monkeypox Variant Emerges in the DRC
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
Rare new drug for obesity! Imcivree, a powerful and first MC4R agonist, is recommended and approved by EU CHMP: weight loss ≥10% after treatment for one year
New drug for obesity: Imcivree is recommended and approved by EU CHMP. Rhythm Pharmaceuticals is a biopharmaceutical company focusing on the development and commercialization of the treatment of rare genetic obesity diseases.
Recently, the company announced that the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) has issued a positive review opinion recommending approval of Imcivree (setmelanotide) for use in children and adults with rare obesity aged ≥6 years. , Treat obesity and manage hunger.
Specifically: Obesity patients confirmed by testing to be caused by pro-opioid melanocytic corticosteroid (POMC), proprotein convertase subtilisin/kexin1 type (PCSK1) or leptin receptor (LEPR) deficiency.
Now, CHMP’s opinions will be submitted to the European Commission (EC) for review, which will usually make an approval decision within the next 2 months. If approved, Imcivree will be the first EU drug to treat these rare genetic obesity
In November 2020, Imcivree was approved by the U.S. FDA and became the first drug in history to treat these rare genetic obesity. In the United States and the European Union, setmelanotide has been awarded Orphan Drug Designation (ODD) for the treatment of POMC and LEPR-deficient obesity, and has been awarded Breakthrough Drug Designation (BTD) and Priority Drug Designation (PRIME) respectively.
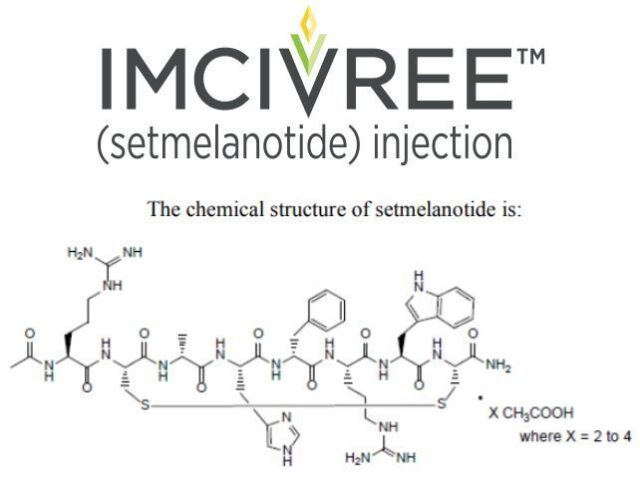
POMC, PCSK1, or LEPR-deficient obesity is an extremely rare disease caused by changes in the POMC, PCSK1, or LEPR genes. These genetic changes damage the melanocortin 4 (MC4) receptor pathway, and the MC4 receptor pathway is the hypothalamus The pathway that regulates hunger, energy expenditure and body weight. Patients with POMC, PCSK1, or LEPR-deficient obesity begin to struggle with extreme and insatiable hunger from an early age, leading to early onset and severe obesity.
The active pharmaceutical ingredient of Imcivree is setmelanotide, which is a pioneering, oligopeptide MC4 receptor agonist designed to restore the function of the damaged MC4 receptor pathway caused by the upstream gene defect of the MC receptor, thus directly solving the root of the disease the reason. Setmelanotide can re-establish the patient’s energy expenditure and appetite control, reduce hunger, and reduce weight.
In phase 3 clinical trials, 80% and 45.5% of obese patients caused by POMC or PCSK1 deficiency and LEPR deficiency, respectively, lost more than 10% of their weight after receiving Imcivree for one year.
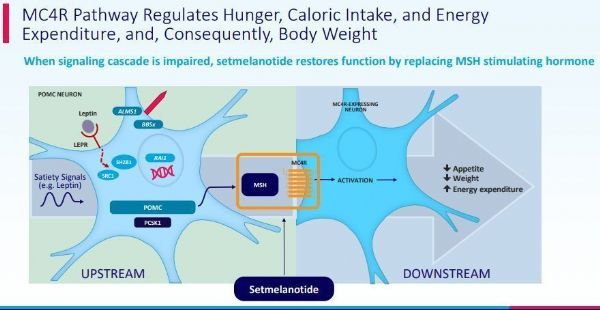
Setmelanotide mechanism of action
CHMP’s positive review opinions are based on the results of two pivotal phase III clinical trials. These two studies evaluated the efficacy and safety of setmelanotide in the treatment of POMC (including PCSK1)-deficient obesity and LEPR-deficient obesity. Both studies reached the primary endpoint and all key secondary endpoints. The data showed that setmelanotide reduced body weight and extreme hunger (hyperappetite) with statistical significance and clinical significance. Importantly, during the withdrawal period (8 weeks), the patient’s body weight and hunger sensation both increased significantly, and after restarting treatment, most patients recovered their weight loss and hunger response. These data confirm setmelanotide It can help restore the function of MC4R pathway in regulating body weight and controlling appetite, and verify its clinical therapeutic effect.
——POMC test data showed that 80% (n=8/10) of POMC-deficient obese patients achieved a weight loss of >10% (p<0.0001) within about a year, and 50% of patients self-reported their hunger score The improvement reached or exceeded 25% (p=0.0004), the weight was reduced by an average of 25.6% from the baseline (p<0.0001), the most hungry rating was reduced by an average of 27.1% from the baseline (p=0.0005), and the average weight loss was 31.2 kg after one year of treatment ( 70.5 pounds).
——LEPR test data showed that 45.5% (n=5/11) of LEPR-deficient obese patients achieved a weight loss of >10% (p=0.0001) in about a year, and 73% of patients self-reported an improvement in their hunger score Reached or exceeded 25% (p<0.0001), the weight was reduced by an average of 12.5% from the baseline (p<0.0001), the most hungry rating was reduced by an average of 43.7% from the baseline (p<0.0001), and the average weight loss was 16.7 kg (36.8) after one year of treatment lb).
In both studies, patients aged 6-17 years (n=14) had a significant decrease in BMI at baseline. Since these patients have not yet completed their growth, appropriate progression of puberty and an increase in height were observed during the study period.
In addition, both studies included a 4-week placebo withdrawal period to further illustrate the effect of setmelanotide treatment. During this period, patients almost immediately gained weight, increased hunger, and reversed the first 12 weeks of weight loss and decreased hunger scores. the trend of.
Consistent with previous clinical experience, setmelanotide was well tolerated in both trials. Treatment-related adverse events (AE) include injection site reactions, nausea and vomiting, headaches, and increased pigmentation (blackening of the skin). No cardiovascular adverse events related to setmelanotide have been reported.
(source:internet, reference only)
Disclaimer of medicaltrend.org



