JAMA : SINOPHARM releases Phase 3 data of two COVID-19 vaccines
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
- Kochi University pioneers outpatient bladder cancer treatment using semiconductor lasers
- ASPEN 2024: Nutritional Therapy Strategies for Cancer and Critically Ill Patients
- Which lung cancer patients can benefit from neoadjuvant immunotherapy?
- Heme Iron Absorption: Why Meat Matters for Women’s Iron Needs
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
JAMA : China SINOPHARM releases phase 3 clinical data of two COVID-19 inactivated vaccines
JAMA : SINOPHARM releases Phase 3 data of two COVID-19 vaccines. Two SINOPHARM inactivated vaccines can produce high-titer antibodies and form effective protection 14 days after two injections.
On May 26, the international medical journal “Journal of the American Medical Association” JAMA published the results of the Phase III clinical trial of the COVID-19 inactivated vaccine published by Sinopharm Sinopharm, titled: Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID- 19 Infection in Adults.
This is the world’s first officially published Phase III clinical trial results of a COVID-19 vaccine inactivated, and this is the first time the results of a Phase III clinical trial of China’s COVID-19 vaccine have been published.
Based on the results of the Phase Ⅲ clinical trial of SINOPHARM COVID-19 Inactivated Vaccine, the study summarized and analyzed the effectiveness and safety of the Beijing Institute of Biological Products (BIBP) and Wuhan Institute of Biological Products (WIBP) of SINOPHARM inactivated vaccines III Phase clinical trial results.
The results of the study show that the two new inactivated vaccines of SINOPHARM can produce high-titer antibodies and form effective protection 14 days after two injections, and the positive conversion rate of neutralizing antibodies in the whole population has reached more than 99%. The protective effect of the WIV04 vaccine group was 72.8%, and that of the HB02 vaccine group was 78.1%. The safety is good, and the adverse reactions are mostly pain at the injection site, which is mild, transient and self-limiting.

The study is a randomized, double-blind, placebo-controlled phase III clinical trial. The main result of the study is the effectiveness of preventing symptomatic COVID-19 14 days after the second dose of vaccine. The secondary result is that the second dose of vaccine is at least The occurrence of severe COVID-19 cases or deaths after 14 days.
The study included 40,382 adult participants who were over 18 years old and had no history of COVID-19. The average age was 36.1 years old, and the proportion of men was 84.4%.
Among them, 13,459 people received the WIV04 vaccine, 13,465 people received the HB02 vaccine, and 13458 people were the placebo control group. The participants received 2 intramuscular injections in a 21-day interval.
As of December 20, 2020, there have been 962 suspected cases after the first injection. After review by the Endpoint Judgment Committee, a total of 255 confirmed cases were obtained, of which 142 confirmed cases were in the monitoring period (14 days after the second injection), and 113 confirmed cases were outside the monitoring period (the first injection to the 35th day) ).
During the monitoring period, there were 26 COVID-19 cases in the WIV04 group, 21 COVID-19 cases in the HB02 group, and 95 cases in the control group. Compared with the control group, the protective effect of the WIV04 group was 72.8%, and the protection of the HB02 group The effectiveness is 78.1%.
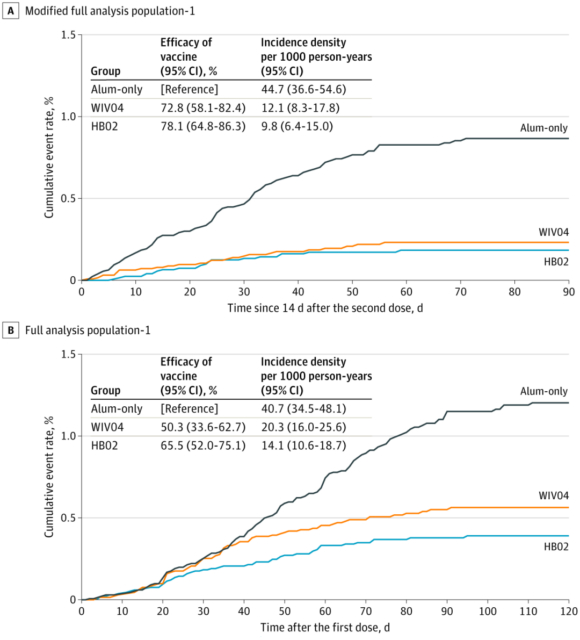
The efficacy of two inactivated vaccines in preventing symptomatic COVID-19
However, after two doses of the vaccine, 47 asymptomatic COVID-19 cases were found, including 16 in the WIV04 group, 10 in the HB02 group, and 21 in the control group. If calculated based on this, the protective efficacy of the WIV04 vaccine is 64.0%. The vaccine efficacy of the HB02 vaccine is 73.5%.
During the analysis period, a total of two severe COVID-19 cases occurred, both in the placebo control group. Therefore, during the analysis period, the effectiveness of the two vaccines in preventing severe COVID-19 both reached 100%.
In terms of neutralizing antibody levels, the geometric mean titer of participants in the two vaccine groups at baseline was 2.3, and that of participants in the control group was 2.4. 14 days after completing two doses of vaccination, the geometric mean titer of the WIV04 group was 94.5 (95 %CI, 89.7-99.5), 156.0 (95% CI, 149.6-162.7) in the HB02 group, and 2.7 (95% CI, 2.6-2.8) in the control group. The relevant data is similar to the data published in the Phase 1/2 clinical trial . The seroconversion rate of participants who received both types of vaccines was almost 100%, while that of the control group was 2.3%.
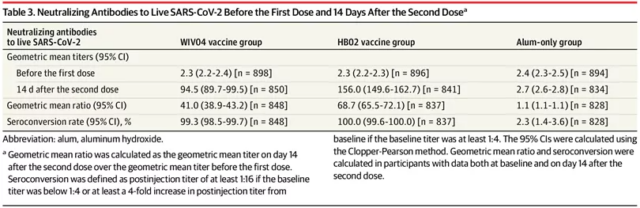
Neutralizing antibody levels and seroconversion rate data
More than 40% of the participants in the three groups had adverse reactions. Among them, the data in the WIV04 group was 44.2%, the data in the HB02 group was 41.7%, and the control group was 46.5%. The main manifestations were pain at the injection site, headache, and most adverse reactions. The severity is mild and no special treatment is required.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



