Immunomodulatory of lactic acid in inflammation/tumor microenvironment
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
The immunomodulatory effect of lactic acid in inflammation and tumor microenvironment
The immunomodulatory effect of lactic acid in inflammation and tumor microenvironment. The tumor microenvironment can inhibit tumor immunity and promote tumorigenesis; the microenvironment of chronic inflammatory tissue promotes inflammation and delays the regression of inflammation.
Although the immunological status of the two environments is quite different, their metabolic status is similar: both are hypoxic, with elevated levels of lactic acid and other metabolic by-products, and low nutrient content.
In 2020, Certo, M et al. published a review titled “Lactate modulation of immune responses in inflammatory versus tumour microenvironments” in “Nature reviews. Immunology“, describing that compared with normal tissues, lactic acid is in tumor and inflammation. The difference in biological activity in the microenvironment contributes to the establishment of a specific immune state in diseases. The brief introduction is as follows:
Cancer and immune-mediated inflammatory diseases (IMIDs), such as rheumatoid arthritis and multiple sclerosis, have a similar metabolic microenvironment, but the immune status of the two is different. Tumor microenvironment (TME) accelerates the metabolism of tumor cells and cancer-related fibroblasts to form an immune environment that promotes tumor growth.
The tumor tissue exhausts local energy, forcing neighboring immune cells to process high-concentration metabolites such as lactic acid in the absence of nutrients, leading to immunosuppression and tumor growth. Compared with TME, the microenvironment of inflammatory diseases such as arthritic synovium has a high level of inflammatory cell subpopulations, such as TH1 cells, TH17 cells, inflammatory macrophages, and CD4+CD25+ with impaired functions.
Treg cells, inflammatory cells maintain the continued existence of the pathological process of IMID. In IMIDs, lactic acid is produced by mesenchymal fibroblasts and metabolically active infiltrating immune cells, and is a key driver of the microenvironment of inflammatory tissues.
The modern view believes that lactic acid is an important carbon source and signal molecule for cell metabolism. Cells in the microenvironment of TME and IMID have completely different responses to lactic acid, so new research in this context has a variety of clinical application potentials.
Lactic acid controls the immune response
The following introduces some metabolic pathways regulated by high lactate levels, which are related to the occurrence and development of chronic inflammatory diseases and cancer.
Lactate dehydrogenase
Lactate dehydrogenase exists in two different isomers, LDHA and LDHB, and can be assembled into homotetramers or heterotetramers in five different combinations. LDHA converts pyruvate into lactic acid and NAD+, while LDHB converts lactic acid into pyruvate to promote oxidative metabolism.
LDHA promotes the effector function of T cells by increasing the acetylation and transcription of interferon-γ (IFNG) and plays a key role in inflammation. The proliferation of effector T cells such as cytotoxic T cells and the production of cytokines are highly dependent on glycolysis, and therefore become inactive under conditions of low glucose levels and high lactic acid concentrations. Treg cells rely less on glycolysis, but rely on oxidative phosphorylation to produce energy. Therefore, lactic acid treatment reduces effector T cell function without affecting Treg cell function. After inhibiting LDH, even in the presence of lactic acid, the function of effector T cells can be restored, which indicates that the effect of lactic acid on these immune cells is highly dependent on LDH.
Inhibitor FX11 inhibits LDHA activity of macrophages, and can down-regulate pro-inflammatory cytokines, inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2) by inhibiting mitogen-activated protein (MAP) kinase phosphorylation , Thus playing an anti-inflammatory effect. The same LDH inhibitors can reduce ATP levels and induce oxidative stress and cell death in cancer.
Some studies support the role of lactic acid and LDHA in tumor progression. After LDHA is knocked out, pan02 cancer cell Pan02 inhibits tumorigenesis in C57BL/6 mice; in the same model, natural killer (NK) cells in tumor tissue depleted by LDHA show higher killing activity, indicating LDH activity The increase in tumor immune escape by suppressing the function of immune cells.
LDH is a cytoplasmic enzyme, but it can also bind to mRNA in the nucleus, indicating that LDH plays a role in the post-transcriptional modification of gene expression. For example, LDH binds to the RNA encoding granulocyte macrophage colony stimulating factor (GM-CSF) rich in AU elements. Therefore, LDH can promote tumor growth through its enzymatic effect or gene regulation function.
PKM2
Pyruvate kinase (PK) is a glycolytic enzyme that catalyzes the irreversible dephosphorylation of phosphoenolpyruvate to pyruvate to produce ATP. Among the various isoforms of PK, PKM2 has received special attention due to its multiple functions in immune cells and cancer cells. The dimeric form of PKM2 can direct glucose metabolism to biosynthetic pathways, including the pentose phosphate pathway, which can act as a kinase to enter the nucleus to regulate transcription, and can also maintain mitochondrial function by binding to the outer mitochondrial membrane.
PKM2 regulates the metabolism and function of immune cells through its role in the Warburg effect. PKM2 expression increases in several inflammatory diseases, such as active Crohn’s disease and sulfonic acid-induced colitis in the intestinal tissues of mice. The dimeric form of PKM2 is increased in macrophages activated by lipopolysaccharide (LPS), which can regulate HMGB1, which in turn stimulates pro-inflammatory cytokines.
Nuclear PKM2 can phosphorylate transcription factor signal converter and transcription activator 3 (STAT3), and promote the production of IL-6 and IL-1β. N,N’-Diarylsulfonamide (DASA) can stabilize PKM2 tetramer, inhibit the translocation of dimer PKM2 to the nucleus, and reduce the expression of IL-17. Another PKM2 nuclear translocation inhibitor TEPP-46 reduces the polarization of TH17 cells and the occurrence of experimental autoimmune encephalomyelitis (EAE).
In cancer cells, PKM2 dimer can translocate into the nucleus to stabilize HIF1α and induce the expression of glycolytic genes. PKM2 can interact with many cytokines. It interacts with the RNA binding protein HuR, regulates the subcellular localization of HuR, cell cycle progression and glioma cell growth, and interacts with the AU-rich protein melamine (TTP).
Destroy the stability of TTP through proteasome degradation to regulate the proliferation of breast cancer cells. PKM2 can enter the mitochondria, phosphorylate and stabilize BCL-2, and promote cancer cells to adapt to oxidative stress. The interaction of PKM2 with NF-κB and HIF1α in the nucleus is the reason for the increased secretion of VEGF-A and the formation of blood vessels during tumor growth.
PKM2 promotes the recruitment of tumor-associated macrophages and myeloid-derived suppressor cells to TME by inducing the release of chemokines, such as CCL8, CCL2 and CXCL1. These cells exert immunosuppressive functions by inducing Treg cells and inhibiting the function of NK cells.
The dimer PKM2 can also regulate the expression of PDL1 in tumor-related immune cells by binding to the hypoxia response element in the PDL1 promoter, thereby creating an immune escape tumor environment.
MAVS
Mitochondrial antiviral signal protein (MAVS) mediates the activation of NF-κB and interferon regulatory factor 3 (IRF3) in response to viral infections, followed by the expression of type I interferons such as IFNβ. The cascade leading to interferon production begins with the activation of innate immune receptors, including retinoic acid-inducible gene I (RIG-I)-like receptors and Toll-like receptors. MAVS directly binds to lactic acid and is inactivated, inhibiting the signal activation of RIG-I-like receptors and the production of type I interferons.
MAVS binds to mitochondria through hexokinase 2 (HK2) to promote glycolytic pathways. After the activation of RIG-1-like receptors, the binding of MAVS to HK2 decreases, and the binding to RIG-1 increases, which releases and inactivates HK2 from mitochondria. Lactic acid makes MAVS no longer able to bind to mitochondria, and the formation of RIG-I-MAVS complex and the production of interferon are inhibited. Given that type I interferon plays a key role in regulating immune cells (such as dendritic cells, Treg cells and cytotoxic T cells), this may be a mechanism by which lactic acid promotes immune surveillance suppression in TME.
In the case of chronic inflammation, lactic acid treatment increases HK2 binding sites on the surface of CD4+ T cell mitochondria. The voltage-dependent binding of HK2 to the anion channel of the outer mitochondrial membrane can promote cell survival, which may explain the persistence and survival of T cells infiltrating chronic inflammatory tissues (such as rheumatoid arthritis synovium).
Fatty acid synthesis
Fatty acid synthesis (FAS) plays a key role in regulating the activity of immune cells. After the activation of immune cells, the energy mainly comes from glycolysis, which leads to an increase in the level of pyruvate and produces and secretes large amounts of lactic acid. Pyruvate can also be converted into acetyl-CoA and citric acid in the mitochondria. Citrate is exported to the cytoplasm and is the main raw material of FAS.
Lipid metabolism regulates the polarization of macrophages to the inflammatory phenotype, and fatty acid oxidation or gluconeogenesis promotes the anti-inflammatory function of M2 macrophages. But in M1 macrophages, the increase in glycolysis is critical, not only can produce ATP faster, but also can obtain citric acid through the tricarboxylic acid (TCA) cycle to maintain the new FAS. In a mouse model of diet-induced inflammation, knocking out fatty acid synthase (Fasn) resulted in a decrease in the number of macrophages recruited into adipose tissue.
During T cell activation, genes that regulate fatty acid biosynthesis are up-regulated. The up-regulation of acetyl-CoA carboxylase 1 (ACC1) and FASN activity increases the pathogenicity of TH17 cells. Soraphen A, an inhibitor of ACC1, can block the differentiation of CD4+ T cells into TH17 cells, which is conducive to the differentiation of T cells into FOXP3+ Treg cells. This mechanism can reduce the severity of EAE. A similar concentration of lactic acid caused CD4+ T cells to up-regulate the de novo synthesis of fatty acids, resulting in increased IL-17 production and decreased cell viability.
Normal cells mainly rely on the uptake and oxidation of fatty acids. However, in tumor cells, even the presence of lipids in the microenvironment will reactivate FAS. This indicates that this pathway plays a key role in their metabolism. FAS activated in cancer cells will change their membrane composition and fluidity and become more resistant to chemotherapy. The activation of FAS in immune cells can also lead to changes in the functions of these cells in TME, such as the reduction of the immune stimulating ability of tumor dendritic cells. In view of the fact that FAS regulates multiple cellular processes in inflammatory diseases and cancer, the regulation of this metabolic pathway has brought great promise for treatment.
Lactic acid shuttle function
Lactic acid shuttles between energy-producing and energy-consuming cells and plays a vital role in physiology, including as an important
Energy sources, gluconeogenesis precursors and signaling molecules. For example, in skeletal muscle, fast-twitch fibers glycolysis and produce lactic acid, and then enter the slow-twitch fiber cells. Similarly, in the metabolic coupling of brain neuronal glial cells, astrocytes output lactic acid as an energy source for neighboring neurons through glycolysis. Part of the carrier transport of lactic acid from glycolytic cells (fast-contracting fibers and astrocytes) to oxidizing cells (slow-contracting fibers and neurons) is provided by the specialized transport of solute carriers (called lactic acid shuttle). The various functions of lactic acid in the microenvironment of inflammatory diseases and TMEs are usually opposite.
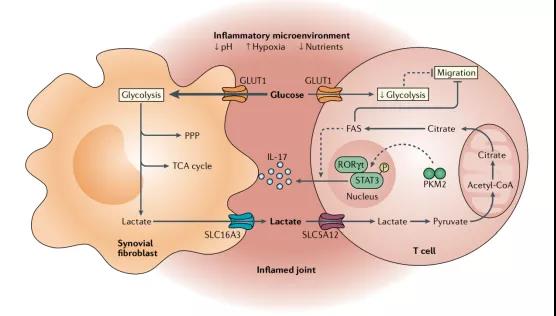
Figure 1 Immune regulation of lactic acid in the microenvironment of inflammatory diseases—taking arthritis synovium as an example
Immune Regulation of Lactic Acid in the Microenvironment of Inflammatory Diseases
In the context of inflammatory diseases, lactic acid triggers a series of intracellular signals to promote chronic inflammatory processes. The lactate-mediated response to delayed hypoxia does not appear to be coupled with HIF1α because it uses NDRG family member 3 (NDRG3). NDRG3 degrades in a PHD2/VHL-dependent manner under normoxia, similar to HIF1α. However, under long-term hypoxic conditions, it is protected from degradation by binding to lactic acid. The increase in NDRG3 levels leads to the activation of the RAF-ERK signaling pathway, which regulates hypoxia-related pathophysiological reactions, including inflammation and angiogenesis. The pharmacological or genetic inhibition of LDHA limits the accumulation of NDRG3 protein in a dose-dependent manner.
In the arthritic synovium, lactic acid regulates the function of immune cells in many ways, namely migration and cytokine production (Figure 1). In T cells, lactate induces “stop migration” signals through CD4+T cell lactate transporter SLC5A12 and CD8+T cell SLC16A1 (MCT1), causing them to accumulate in the inflammation site. Lactic acid-mediated inhibition of T cell movement in inflamed tissues is combined with reduced glycolysis. Sodium lactate inhibits the expression of several glycolytic enzymes of CD4+ T cells and reduces the expression of glucose flow, so that T cells accumulate in the inflammation site. In rheumatoid arthritis, the initial CD4+ T cells inhibited the intracellular glycolysis level due to the down-regulation of 6-phosphofructose-2-kinase/fructose-2,6-bisphosphatase (PFKFB3), resulting in 6-phosphate glucose (G6P) Shunt to the pentose phosphate pathway to produce NADPH, which changes the activation of telangiectatic ataxia mutant protein (ATM), which is an important enzyme involved in cell cycle regulation. In short, these changes lead to the active proliferation of CD4+ T cells in rheumatoid arthritis and the production of pro-inflammatory sub-cell populations such as TH1 and TH17, leading to chronic inflammation.
ATPlowpyruvatelowNADPHhiCD4+ T cells in rheumatoid arthritis also showed enhanced FAS, leading to accumulation of cytoplasmic lipid droplets and upregulation of TKS5. Therefore, TKS5hiCD4+T cells from patients with rheumatoid arthritis form membrane folds rich in actin and corticin, which increase the ability to infiltrate inflamed tissues. Inhibition of FAS can reverse all of the above effects. FAS not only led to increased infiltration of CD4+ T cells at the site of inflammation, but also led to their retention in the site.
In the arthritic synovium, the activation of specific metabolic pathways induced by lactic acid or SLC5A12 promotes the infiltration and differentiation of CD4+ T cells into TH17 cells and T follicular helper cell subsets, and induces the formation of different lymphatic structures in the same inflammatory environment. It plays a vital role in the pathogenesis of rheumatoid arthritis.
In mouse arthritis models, inhibition of SLC5A12 reduced the uptake of lactic acid, restored T cell function and reduced inflammation, indicating that it may be a new target for the treatment of inflammation in IMIDs. The increased metabolic demand of synovial cells leads to the accumulation of lactic acid in the synovial fluid of patients with rheumatoid arthritis. The level of lactic acid in the synovial fluid is a reliable indicator to distinguish between inflammatory arthritis and septic arthritis. Compared with patients with osteoarthritis, patients with rheumatoid arthritis have higher levels of LDH isoenzymes in serum and synovial fluid, and the activity of LDH in synovial tissues is higher than that in healthy controls. Lactic acid can activate Gi protein-coupled receptor 81 (GPR81), inhibit cAMP and protein kinase A (PKA) signal transduction and β-arrestin activation. By activating this mechanism in monocytes and macrophages, lactic acid can reduce inflammation in pancreatitis and hepatitis models.
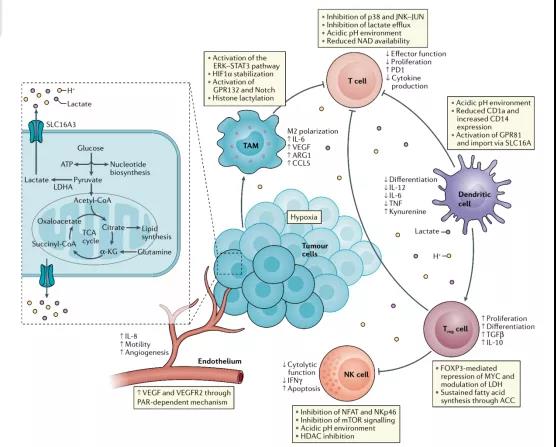
Figure 2: Immune regulation of lactic acid in TME
Immune Regulation of Lactic Acid in TME
In 1923, Otto Warburg first observed the aerobic glycolysis of cancer cells. Even in the presence of sufficient oxygen, it also showed high sugar uptake and excessive lactic acid formation. This is called the Warburg effect, and it is still the top ten cancer cell. One of the characteristics. The lactic acid produced by cancer cells is secreted into the extracellular environment to promote cancer progression.
The efflux of lactic acid coupled with protons in cancer cells or stromal cells can regulate TME (including cell invasion, angiogenesis, survival signals, metastasis development and evasion of immune surveillance) to promote tumor progression. Extracellular acidosis inhibits T cell-mediated immunity, and neutralizing tumor acidity can improve the anti-tumor effect of immunotherapy.
The extracellular pH value between 6.0-6.5 induces tumor-specific CD8+ T cells to become anergy, and their cytolytic activity and the ability to secrete cytokines are reduced. Proton pump inhibitor treatment can effectively restore T cell function.
The low pH in TME reduced the expression of iNOS, CCL2 and IL-6 in M1 macrophages, and increased the expression of M2 macrophage markers. Extracellular acidification also inhibits the anti-tumor activity of NK cells through mTOR.
Cells such as cancer cells, T cells, NK cells, dendritic cells, and macrophages in TME can sense the level of extracellular lactate, which triggers intracellular signals, fine-tunes cell behavior and strongly affects their functions (Figure 2). The activation of GPR81 induced by lactic acid is involved in tumor growth and regulates the expression of genes related to lactic acid uptake and metabolism. Lactic acid can activate G protein-coupled receptor 132 (GPR132) in macrophages and promote the polarization of macrophages to M2.
The lactoyl group derived from lactic acid can be used for post-translational modification of histones to increase the expression of IL6 and ARG1 genes. Lactic acid inhibits the differentiation of monocytes into dendritic cells, and inhibits the production of cytokines and cytotoxic activity of T cells. The lactic acid in TME reduces the cytolytic function of NK cells and increases the number of myeloid suppressor cells. Lactic acid can prevent the activation of NFAT in T cells and NK cells and reduce the production of IFNγ, which is contrary to the effect of lactic acid in promoting IFNγ production in an inflammatory environment.
The expression of LDHA is regulated by HIF1α, MYC and p53, which can promote epithelial-mesenchymal transition and increase angiogenesis and invasion. Down-regulation or absence of LDHB expression is an important early event in the development of prostate cancer, breast cancer and pancreatic cancer, which is related to high proliferation, easy infiltration and poor prognosis of patients. In the absence of glucose, LDHB-mediated lactic acid use supports autophagy to maintain metabolism and cancer cell growth.
Changes in LDHB expression are often related to early metabolic adaptation, indicating that the production and use of lactic acid may participate in the metabolic adaptation of tumor cells to support metastasis. Bone metastatic breast cancer cells release large amounts of lactic acid. Lactic acid is a key energy source for osteoclasts. Osteophilic tumor cells can release lactic acid to promote osteoclast differentiation and metabolic reprogramming, allowing tumor cells to invade and metastasize.
Lactic acid stimulates endothelial cells to release VEGF to promote wound healing and tumor-related angiogenesis. In glioma cells, lactic acid induces the expression of transforming growth factor β2, which is a key regulator of cancer cell migration, invasion, epithelial-mesenchymal transition and metastasis formation. In summary, the expression of lactate and LDH can support the metabolic adaptation of tumor cells and tumorigenesis.
Expert Comments:
Cancer and IMIDs have similar metabolic microenvironments but their immune status is different. The accelerated metabolism of tumor cells and cancer-related fibroblasts in TME forms an immune environment that promotes tumor growth.
The tumor tissue exhausts local energy, forcing neighboring immune cells to process high-concentration metabolites such as lactic acid in the absence of nutrients, leading to immunosuppression and tumor growth. Compared with TME, the microenvironment of inflammatory diseases such as arthritic synovium has a high level of inflammatory cell subpopulations, such as TH1 cells, TH17 cells, inflammatory macrophages, and CD4+CD25+ with impaired functions.
Treg cells, inflammatory cells maintain the continued existence of the pathological process of IMID. In IMIDs, lactic acid is produced by mesenchymal fibroblasts and metabolically active infiltrating immune cells, and is a key driver of the microenvironment of inflammatory tissues.
The modern view believes that lactic acid is an important carbon source and signal molecule for cell metabolism. Cells in the microenvironment of TME and IMID have completely different responses to lactic acid, so new research in this context has a variety of clinical application potentials.
Understanding the mechanism by which local metabolites control the immune response in diseased tissues of cancer and IMIDs has great therapeutic significance. In-depth basic research is for better clinical care.
Targeting specific metabolic pathways is becoming a useful and promising therapeutic strategy in IMIDs, and future research on immune metabolism is expected to provide new drugs that can target and regulate immune cell activity with fewer side effects.
Immunomodulatory of lactic acid in inflammation/tumor microenvironment
Immunomodulatory of lactic acid in inflammation/tumor microenvironment
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



