Avatinib is approved for cytosis and Continuous relief more than 3 years!
- Did Cloud Seeding Unleash a Deluge in Dubai?
- Scientists Identify Gut Bacteria and Metabolites that Lower Diabetes Risk
- OpenAI’s Model Matches Doctors in Assessing Eye Conditions
- UK: A Smoke-Free Generation by Banning Sales to Those Born After 2009
- Deadly Mutation: A New Monkeypox Variant Emerges in the DRC
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
Avatinib is approved for cytosis and Continuous relief more than 3 years!
Avatinib is approved for cytosis and Continuous relief more than 3 years! Avatinib is approved for the treatment of cytosis! Continuous relief for more than 3 years!
Avatinib has brought significant clinical benefits to SM patients, and it is expected to change the precision treatment model of this disease and benefit more SM patients.
Recently, the US FDA announced that it has approved Avapritinib (Avapritinib, trade name: Ayvakit) as a new indication for the treatment of adult patients with advanced systemic mastocytosis (SM), including aggressive SM (ASM). ), SM (SM-AHN) and mast cell leukemia (MCL) with hematological tumors.
This is the first approved targeted therapy for patients with advanced SM, which aims to effectively and selectively inhibit the KIT tyrosine kinase (the core driver of the disease) carrying the D816V mutation.
▌Mastocytosis (SM)
Mastocytosis (SM) is a rare blood system disease, mainly driven by the KITD 816V mutation.
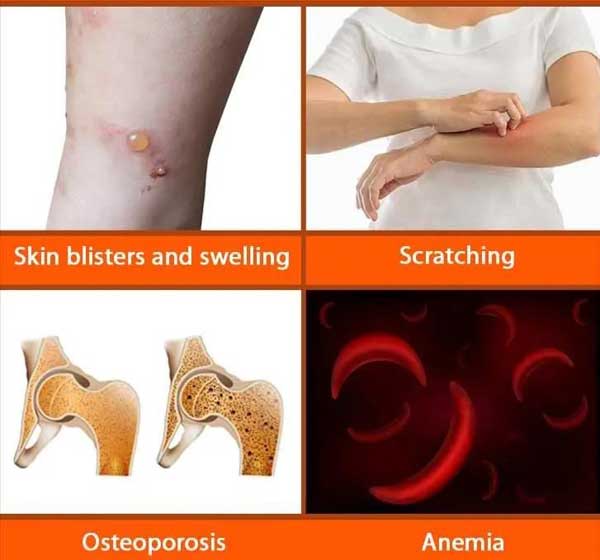
The uncontrolled proliferation and activation of mast cells can cause extensive damage to multiple organ systems in patients with SM. The disease can occur at any age, and the clinical manifestations vary.
Late SM subtypes include ASM, SM-AHN and MCL. Among them, the median overall survival of ASM patients is about 3.5 years, SM-AHN is about 2 years, and MCL patients are less than half a year.
Even with a variety of symptomatic treatments, debilitating symptoms such as allergic reactions, maculopapular rash, itching, diarrhea, brain fog, fatigue, and bone pain continue to exist in all types of SM, resulting in limited work and daily activities of patients. Faced with both physical and mental pressure.
Previously, there was no approved therapy that can selectively inhibit the KIT D816V mutation, and new treatment options are urgently needed to solve these life-threatening complications.
▌Broad-spectrum KIT/PDGFRA inhibitor: avatinib
Avatinib is a kinase inhibitor (KIT), which can selectively and accurately inhibit KIT and PDGFRα mutant kinases, and has a broad inhibitory effect.
Currently, two indications have been approved by the FDA:
① Adult patients with unresectable or metastatic gastrointestinal stromal tumor (GIST) carrying PDGFRA exon 18, including PDGFRA D842V mutation.
② Adults with advanced SM, including ASM, SM-AHN and MCL, and PDGFRα D842V.
In March of this year, Avatinib was approved by the National Food and Drug Administration (NMPA) for the indication of Avatinib: for the treatment of platelet-derived growth factor receptor (PDGFRα) exon 18 mutations (including PDGFRα D842V mutations) ) In adult patients with unresectable or metastatic GIST.
▌The effect is long-lasting, and the clinical trials are amazing!
This FDA approval is based on positive data from the Phase 1 EXPLORER trial and the Phase 2 PATHFINDER trial.
The results show that:
- Avatinib has shown long-lasting clinical efficacy in advanced SM patients with different disease subtypes, regardless of previous treatment.
- At a median follow-up period of 11.6 months, the total response rate (ORR) of 53 evaluable patients was 57%, and the complete response/hematological complete response rate (CR/CRh) was 28%. The median duration of remission was more than three years (38.3 months).
It should be noted that affatinib is not recommended for advanced SM patients with low platelet counts (less than 50,000/μL).
Patients with advanced systemic mastocytosis usually have some life-threatening complications or debilitating symptoms that change their ability to perform daily activities.
And avatinib has brought significant clinical benefits to SM patients, which is expected to change the precision treatment model of this disease and benefit more SM patients.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



