Flexible ureteroscope high pressure perfusion: Impacts on kidney histology
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
Flexible ureteroscope high pressure perfusion: Impacts on kidney histology
Flexible ureteroscope high pressure perfusion: Impacts on kidney histology. The histological effects of ureteroscopy on human kidneys are still unclear.
Purpose:
High intrarenal pressure during flexible ureteroscopy is related to unfavorable renal tissue changes and renal pelvic venous reflux. Our purpose is to study the influence of various intra-renal pressure changes on renal histological changes and fluid extravasation during simulated ureteroscopy.
Materials and Method:
The kidneys of 24 young pigs with intact ureter were intubated with Olympus flexible ureteroscope (with or without ureteral access sheath), and the perfusion pressure was set to 50 mm, 100 mm, and 200 mm Hg for perfusion saline irrigation under constant pressure 30 minutes. Kidney tissue samples were collected, processed, and stained, and an unsuspecting pathologist assessed the extent of water penetration into the renal parenchyma as a percentage of the total parenchymal thickness from the urothelium to the renal capsule.
Results:
When the perfusion pressure is set to 50, 100, and 200 mmHg, without the ureteral access sheath, the average percentage of renal tissue penetration with water in the cortical tubules is 33.1, 31.0, and 99.3%, respectively, while in the presence of ureteral access The case of the sheath is 0, 0 and 18.8%, respectively. Overall, kidneys with access sheaths showed lower average tissue penetration under all pressures compared to kidneys without sheaths (6.3% vs 54.5%, p=0.0354). Among the kidneys with a sheath, 11% of the kidneys showed signs of water, compared with 56% of the kidneys without a sheath.
In conclusion:
Pressurized endoscopic irrigation causes a large amount of extravasation of fluid into the renal parenchyma. In the isolated pig model, during ureteroscopy, higher intrarenal pressure is associated with increased perfusion fluid penetration.
Preface
Ureteroscopy, percutaneous nephrolithotomy, and hydronephrosis in the obstructive system may cause high intrarenal pressure and may cause renal pelvic venous reflux, and there is urinary fluid communication between the renal fornix and renal veins (1-3). Intrarenal pressure exceeding 20-40 mmHg has been shown to cause renal pelvic venous reflux (4, 5).
During endoscopic surgery that uses a lot of manual irrigation, the intrarenal pressure can be very high, even exceeding 400 mmHg (6). In percutaneous nephrolithotomy, it has been proved that renal pelvic venous reflux caused by high intrarenal pressure is related to postoperative fever (5, 7). A study showed that after ureteroscopy, the incidence of systemic inflammatory response syndrome (SIRS) may be about 8%, and when ureteral access sheath (UAS) is used, a larger diameter may be associated with a lower incidence of SIRS Related (8). Ogg et al. studied the intrarenal pressure during flexible ureteroscopy using a percutaneous nephrostomy tube using a pressure sensor, and found that when the UAS is in place, the average intrarenal pressure of the pelvic ureteroscope is reduced by more than half (94 mmHg vs. 94 mmHg). 41 mmHg). In addition, a larger diameter UAS has been shown to reduce intrarenal pressure and improve irrigation (10).
The purpose of this study is to evaluate how the flushing pressure during an isolated ureteroscopy affects the depth of histological tissue penetration of the collection system flushing fluid in the pig kidney system using ink marks. We hypothesize that higher intrarenal pressure is related to the increase in ink penetration depth, and that the use of UAS will reduce the intrarenal pressure and the degree of tissue penetration. The purpose of these experiments is to provide a basic framework and proof of concept for future research. These studies may choose to use these methods to study renal pelvic reflux and renal pelvic fluid extravasation.
Materials and Method
Kidneys with intact ureters were taken from FDA-grade pigs executed on the same day. Pigs are between 5 and 6 months old and weigh 73 kg on average. They are males. All boars were castrated within 5 days after birth. The kidney system is freshly obtained from a nearby slaughterhouse. The experiment was carried out within 6 hours after the pig died. The use of isolated porcine kidney system (11) has been proven effective in other experiments that simulate ureteroscopy.
In order to evaluate the influence of intrarenal pressure on fluid extravasation to kidney tissue, we conducted an experiment in which a ureteroscope was retrogradely entered into the ureter-kidney model and fluid was perfused to generate pressure. The kidneys are divided into four pressure groups. In these pressure groups, the external pressure of the irrigation fluid will remain unchanged: 50 mmHg, 100 mmHg, 200 mmHg and no additional pressure. For each pressure setting, three experiments were performed on kidneys using sheaths and three experiments were performed on kidneys without sheaths.
A separate kidney was used for each experiment. A total of 24 kidneys were used-3 for each sheath or unsheathed group in each pressure group. First, insert an 8.4F flexible ureteroscope (Olympus URF Type P5, Olympus, Center Valley, PA) into the ureter, with or without ureteral access sheath. For the sheath experiment, a Flexor (Cook Medical, Bloomington, IN) was used to enter the sheath, the length is 35 cm, and the inner and outer lumen diameter is 12/14F.
In such an experiment, the sheath is advanced to the ureter-pelvic junction (UPJ) and secured in place with a loose circumferential 1-0 ribbon around the distal ureter to prevent the sheath from slipping into the distal ureter and maintain their position. In the experiment without the access sheath, the ureter was also fixed on the ureteroscope with the tip located in the renal pelvis.
For these experiments, the goal is not to simulate ureteroscopy (without ureteroscopy), but to reliably produce consistent intrarenal pressure. Physiological saline is mixed with black ink to make a 1.0% solution. The irrigation fluid is directly connected to the 3.6F working channel of the ureteroscope. The pressure is generated using the pressure wrapped around a new 3 liter saline ink bag.
The pressure gauge is constantly adjusted to maintain a constant pressure of 50, 100, and 200 mmHg. For the kidneys of the control group without additional pressure, a 3L flushing bag was used without pressure, but because the wall tension of the saline bag was placed horizontally with the endoscope channel, it was fluid.
nce the flow is turned on in the kidney, the pressure in each experiment is maintained for 30 minutes. Use General Electric CardioLab® to continuously monitor intrarenal pelvic pressure using a 5Fr catheter located at the junction of the ureter and renal pelvis, and place it retrogradely along the ureteroscope.
After flushing the perfusion fluid for 30 minutes, the kidney was divided into two parts, and a full-thickness tissue sample was collected from the upper pole and from the urothelium to the lower pole of the kidney capsule. Collect the sample carefully to make it uniform throughout the experiment. The samples were fixed in 10% formalin solution for 7 days. After processing, the samples were embedded in paraffin, cut into 8 micron sections, and stained with hematoxylin and eosin.
The sample is then randomly assigned a study identification code and sent to an unsuspecting urological pathologist, who will evaluate the presence and location of the ink. The penetration depth is determined as the maximum distance from the urothelium to the renal capsule, and the result is expressed as the percentage of movement from the urothelium to the renal capsule.
Depth is expressed as a percentage of the total thickness of parenchyma. Assess whether the tubules are damaged. The Fisher’s exact test of proportions and the Wilcoxon signed rank test of the mean were used for statistical analysis.
Results:
A total of 24 kidneys were used, and the average single kidney weight was 160.3 grams (SD 21.5 grams). Compared to when the sheath was used at all flushing sphygmomanometer pressure settings, higher intrarenal pressure was observed when the sheath was not used (p<0.001 for comparison at each pressure setting) (Table 1). Control kidneys that did not receive pressure flushing showed no tissue penetration of the ink.
When the pressure of the sphygmomanometer is 50, 100, and 200 mmHg, the average percentage depth of the tissue penetration of the ink from the urothelium to the kidney capsule is 33.1, 31.0, and 99.3%, respectively, while without UAS, it is 14.0, and 0 and respectively. 18.8% (Figure 1). Under a sphygmomanometer pressure of 200 mmHg, the penetration force of the inserted sheath was significantly lower than that of the uninserted sheath p=0.046.
Table 1 The intrarenal pressure was observed during various flushing pressures with and without sheath.
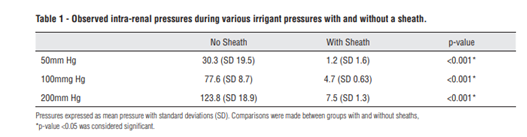
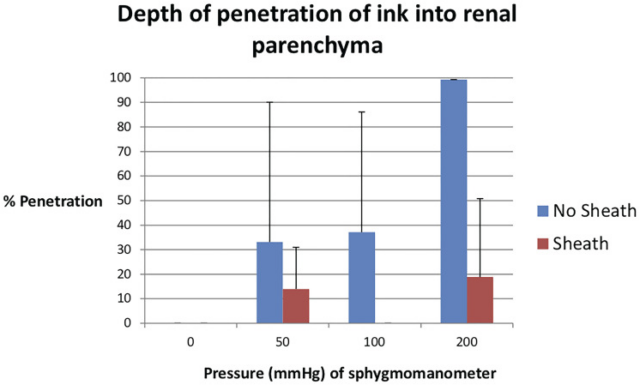
Figure 1 The measurement of ink penetration depth under different pressure settings on the pressure gauge. The data is displayed as the percentage of penetration calculated as the maximum distance of ink infusion to the urothelial tubules divided by the total distance of the urothelium…
Figure 2 A section of the kidney parenchyma stained with hematoxylin and eosin, showing the infusion of ink into the renal tubules. This kidney belongs to the group where the sphygmomanometer is set to 200 mmHg without a sheath. 2A: Low-magnification examination (100x) of renal parenchymal slices,
Measurement of ink penetration depth under different pressure settings on the pressure gauge. The data is shown as the percentage of penetration calculated as the maximum distance of ink infusion to the urothelial tubules divided by the total distance of the urothelium. Overall, kidneys with access sheaths have less average tissue penetration under all pressures than kidneys without sheaths (6.3% vs. 54.5%, p=0.035).
In the absence of UAS, the proportions of the kidneys where any ink enters the cortical tubules at pressures of 50, 100, and 200 mmHg are 0.33, 0.33, and 1.00, respectively, while in the case of UAS, they are 0, 0, and 0.33, respectively . An example of tissue perfusion is shown in Figure 2. Four of the five kidneys showed significant tubular damage involving the medulla, with detachment or displacement of the tubular epithelium, and no sheath.
Discussion:
In this study, we describe a method that can use ink mixtures to measure fluid extravasation into the kidney parenchyma. We show that higher intra-renal pressure is related to an increase in the distance the ink enters the renal parenchyma. This method can provide a basis for future experiments aimed at measuring the impact of urological surgery and its associated pressure on the extravasation of irrigation fluid and renal pelvic venous return. The purpose of this research is not to replicate real ureteroscopy in humans, but to provide a framework for future research to use these methods.
Studies have shown that the degree of renal pelvic venous reflux caused by irrigation and renal tissue damage may depend on intrarenal pressure, which emphasizes the importance of intrarenal pressure during endoscopic surgery. A study by Schwalb et al. It has been found that in minipig kidneys, intrarenal pressure can reach up to 439 mmHg during pelvic ureteroscopy (12). In this study, the histological changes of the kidneys exposed to high pressure were more varied than those of low pressure: acutely, high pressure kidneys showed tubule vacuolation and degeneration, while subacute high pressure kidneys showed evidence of tubular scarring.
However, it is not clear what the long-term clinical significance of these tissue changes may be. The study evaluated the results of patients with mild to moderate renal insufficiency undergoing ureteroscopy and found that the procedure did not seem to be associated with long-term renal impairment (13, 14).
High intrarenal pressure is also associated with infection complications. In a study comparing mini-PCNL with conventional PCNL, mini-PCNL surgery showed higher intrarenal pressure and higher end-organ damage rate <0.001. In addition, persistent high intrarenal pressure and postoperative fever Related to (8). These data indicate that, in some cases, high intrarenal pressure may lead to undesirable consequences.
Our study did not specifically measure bacteria during periods of high intrarenal pressure, but instead used ink as a marker. We believe that this technique of monitoring ink perfusion can be used in experiments, the goal of which is to assess the tissue infiltration of infected fluids and even tumors. Research speculates whether endoscopic surgery for the diagnosis and treatment of upper urethral epithelial cancer will “seed” additional cancer in the upper urethra and cause tumor cells to implant in the kidney parenchyma, but endoscopic monitoring did not show significant changes in clinical results ( 16, 17). Using the methods we demonstrated in our research, the subject has the potential to be further studied in ex vivo or in vivo animal models, using tumor cells and ink to visualize tissue penetration.
We conducted this study to prove the influence of intrarenal pressure on the degree of extravasation of irrigation fluid during endoscopic surgery. Studies have shown that in simulated ureteroscopy using a pig model, high perfusion pressure is associated with a significant increase in the penetration of ink into kidney tissue. We found that the pressure dependence of the ink’s ability to penetrate the inside and outside of the tubule disappeared with the placement of the sheath. By allowing a sheathed irrigation fluid outlet, the intrarenal pressure is lower at the same infusion pressure.
These results provide further evidence for the growing literature that the ureteral access sheath may help protect kidney tissue from damage and systemic infections. Experiments without an access sheath included a lace around the ureter to prevent the ureteroscope from sliding distally, which would almost certainly prevent fluid from flowing around the ureteroscope.
These methods are performed because our goal is to keep the pressure in the kidney relatively constant. Other studies have shown that antegrade leakage of fluid during ureteroscopy is important to help reduce pressure and promote irrigation (18). In our study, as we tried to maintain a constant pressure on the pressure gauge, there may be variable intrarenal pressure.
We used experiments with and without access sheaths because previous studies have shown that the use of sheaths during endoscopic surgery may reduce intrarenal pressure (19). Interestingly, some experiments at 100 mmHg pressure settings or even lower pressure settings did not show ink extravasation. This may be due to the flushing process during the preparation of the paraffinized tissue, and may be a limitation of the use of ink as a marking. For the subsequent experiments that these methods may appear, the influence of pressure regulation on the penetration of a fluid can be studied. A retrospective study comparing ureteroscopy with manual manual irrigation and gravity pressure bag showed that postoperative fever, SIRS, and emergency department visit rates were higher in the manual irrigation cohort (20). It may be that rapid, instantaneous changes in pressure are more likely to cause flushing fluid to flow back.
The histological effects of ureteroscopy on human kidneys are still unclear. Our study used pig kidneys, which have been verified as suitable human kidney models (21, 22). This study has several limitations. We did not perform a real simulated ureteroscopy because the endoscope was not manipulated during the operation, the stones were not actively lasered, and the endoscope was fixed on the ureter to maintain consistent intrarenal pressure.
We only ran each experiment for 30 minutes. Therefore, the procedures in this study do not reflect real-life human ureteroscopy. However, the damage to the urothelium caused by laser or calculus exercise may provide a mechanism for the collection system flushing fluid to penetrate into the renal parenchyma, which we cannot study. In addition, because the ureteroscope is difficult to slip out of the soft non-contracted ureter, we need to gently tie a ribbon around the distal ureter, which may increase the intrarenal pressure of all experimental pressures and reduce the antegrade flow.
Our goal is not to simulate a real human ureteroscopy, but to demonstrate the effect of intrarenal pressure on the tissue penetration of the irrigation fluid and evaluate whether these effects can be mitigated by placing an access sheath. In addition, we tried to collect uniform samples from the upper and lower poles of each kidney after the experiment was completed. An unwitting urological pathologist measured the distance from the urothelium to the tubule closest to the renal capsule through ink penetration. However, slight changes in the way the sample is cut in the slice may change this maximum distance, so a percentage is used instead of the original distance.
Future studies may use real in-vivo flexible ureteroscopy in a pig model to evaluate tissue penetration in a more clinical setting. In future investigations, alternative routes of return can also be evaluated. Here, black ink is a substitute for monitoring fluid extravasation, but since ink molecules will clump and get stuck in small tubes, the total migration of ink may underestimate the extent of fluid penetration in the kidney.
This study shows that in ureteroscopy, the known intrarenal pressure varies greatly (9), and increasing the flushing pressure causes deeper tissue penetration of the ink. In addition, when UAS is not used, the tissue penetration rate of the ink is higher. There is no significant increase in tissue penetration when using the access sheath. We concluded that the technique of using ink in a high intrarenal pressure environment is a reliable method to measure the extravasation of fluid into the kidney tissue.
Cconclusion
The histological effects of ureteroscopy on human kidneys are still unclear. The study showed that in the pig model, the higher saline flushing pressure during simulated ureteroscopy led to an increase in the degree of tissue penetration by renal fluid.
Future research may use ink with markings to monitor fluid extravasation in other clinical situations.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



