SINOVAC Sabin strain inactivated polio vaccine was approved by China
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
- Kochi University pioneers outpatient bladder cancer treatment using semiconductor lasers
- ASPEN 2024: Nutritional Therapy Strategies for Cancer and Critically Ill Patients
- Which lung cancer patients can benefit from neoadjuvant immunotherapy?
- Heme Iron Absorption: Why Meat Matters for Women’s Iron Needs
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
SINOVAC Sabin strain inactivated polio vaccine was approved by China
SINOVAC Sabin strain inactivated polio vaccine was approved by China. SINOVAC (Kexing) Sabin strain inactivated polio vaccine was approved by China, helping to eradicate the last mile of polio globally.
On July 15, 2021, Kexing Holding Biotechnology Co., Ltd. (SINOVAC ) announced that the Sabin strain inactivated polio vaccine (“sIPV”) developed by its subsidiary Beijing Kexing Biological Products Co., Ltd. (“sIPV”) has been released in 2021. On July 12, the drug registration approval document (approval number: S20210023, S20210024) issued by China National Medical Products Administration was obtained, and it is expected to start supplying to the market within this year.
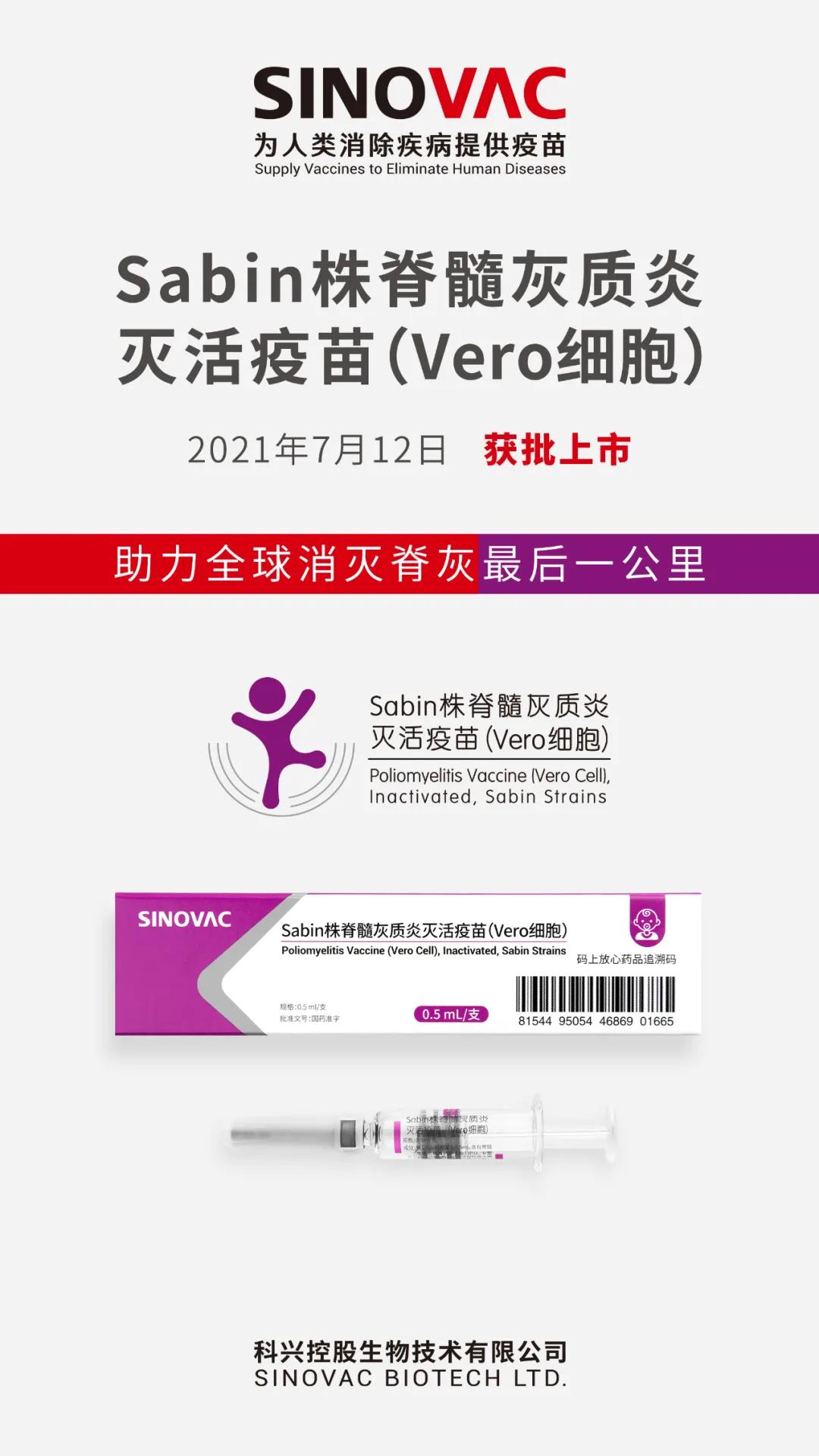
SINOVAC’s sIPV is used in infants and children, mainly for infants and young children of 2 months old and above, to prevent poliovirus types I, II and III caused by poliovirus. The basic immunization program of the vaccine is 3 doses. The first immunization starts at the age of 2 months, and the immunization is 3 consecutive times with an interval of at least 1 month. The booster immunization is once at 18 months. The results of the phase III clinical study showed that the overall adverse reactions of subjects from 0-30 days after vaccination were mainly mild or moderate. There were no serious adverse events or rare adverse reactions related to vaccination, and the safety was good.
Under the influence of the global COVID-19 epidemic, some countries where wild polioviruses are endemic have rebounded. In addition, vaccine-derived polioviruses (Vaccine-derived poliovirus) caused by the circulation of live vaccine viruses in the human body and the external environment have appeared in many countries. Derived Poliovirus (VDPV) and Vaccine-Associated Paralytic Poliomyelitis (VAPP), especially the number of VAPP cases have reached a new high in the past 10 years.
SINOVAC’s sIPV is an inactivated vaccine. There is no risk of paralysis caused by vaccination (VAPP) during the entire vaccination process. People vaccinated with inactivated polio vaccine will not excrete active poliovirus through the digestive tract to the outside environment. Therefore, there is no risk of disease caused by the survival and mutation of vaccine strains in the external environment.
SINOVAC has been focusing on the global polio eradication campaign since the beginning of sIPV research and development, applying the world’s leading technology and using international standards to develop and produce vaccines.
The company’s international cooperation on vaccine research and development and production includes: obtaining vaccine virus seeds and virus culture cell seed lines from the World Health Organization (WHO), importing process technology from Intravacc in the Netherlands, designing production plants, and clinical serum testing.
Professional institutions/organizations work together. sIPV adopts SINOVAC’s international standard quality system and uses bioreactor with microcarrier technology. Compared with traditional processes, it has the characteristics of large scale of cultivation, more precise process control, and low risk of sterile control. The vaccine does not contain any preservatives and antibiotics, thereby reducing the risk of related adverse reactions.
The sIPV developed by SINOVAC has submitted the WHO pre-qualification (PQ) application materials to the WHO in early 2020, and will accept the WHO pre-qualification on-site inspection in February 2021. After passing the WHO pre-certification, the sIPV developed by SINOVAC will work with global public health agencies to jointly promote the global eradication of polio.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



