Effectiveness of the COVID-19 vaccine by neutralizing antibody titers?
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
- Study shows regular seafood consumption may increase risk of exposure to PFAS
- A Persistent Crisis: The Looming Specter of Drug Shortages in United States
- Rabies: The fatality rate nearly 100% once symptoms appear
- Human Brain Continues to Grow: Study Shows Increase in Size and Complexity
- CRISPR Genome Editing: From Molecular Principles to Therapeutic Applications
Predict the effectiveness of the COVID-19 vaccine by neutralizing antibody titers?
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
Effectiveness of the COVID-19 vaccine by neutralizing antibody titers? It is a very feasible method to predict the protective power of the COVID-19 vaccine by neutralizing antibody titers.
The stock prices of Moderna and Novavax have risen a lot because investors accurately predicted the protective effect of vaccines against COVID-19 in their phase 3 clinical trials based on the neutralizing antibody titers in the phase 1 clinical trials of these vaccine companies.
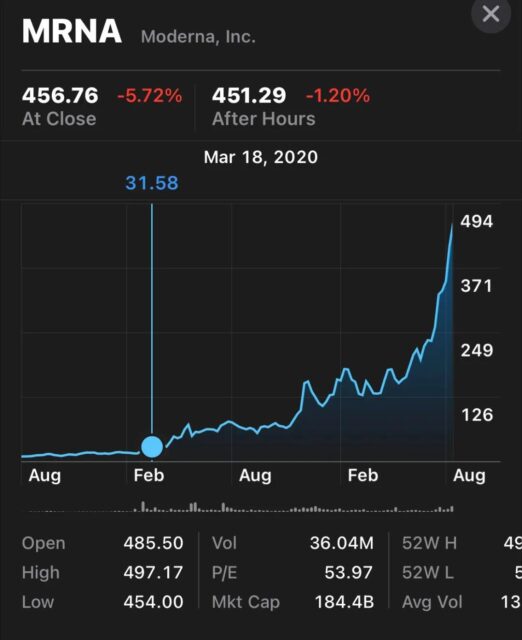
Changes in Moderna’s stock price
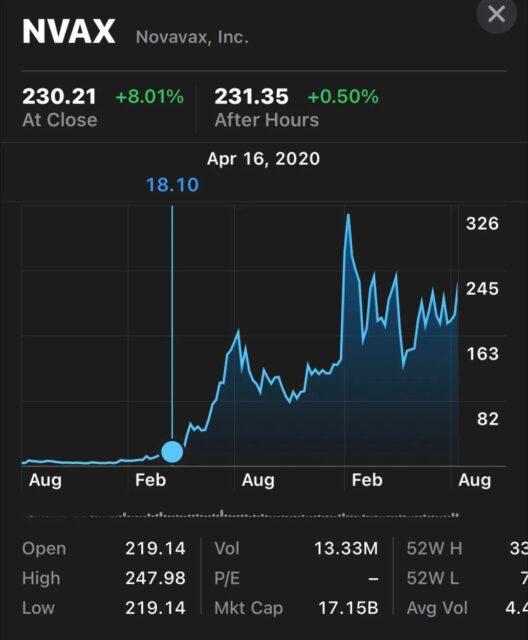
Changes in Novavax’s stock price
.
All currently marketed preventive vaccines basically produce protection by inducing neutralizing antibodies.
But this is just “common sense.” For the COVID-19 vaccine, it needs to be verified by rigorous scientific research.
On August 10, 2021, Moderna, the National Institutes of Health (NIH) VRC, and the Fred Hutch Institute jointly uploaded a very important pre-printed article on medRxiv, which is also the most important COVE of Moderna’s COVID-19 vaccine A recent result of the study (NCT04470427).
This study analyzed the correlation between the protective power of mRNA-1273 and the immune response induced, so it immediately attracted widespread attention.
The study showed that the phase 3 clinical protection of mRNA-1273 was 94%. The study found that 57 days after the first vaccination (4 weeks after the second vaccination), the vaccine-induced RBD and S-specific IgG, as well as the serum neutralization titer, and the occurrence The risk of COVID-19 is inversely proportional (p = 0.003-0.01).
The higher the serum neutralization titer (cID50) of the 57-day vaccinators, the lower the risk of infection in the next 100 days. The cID50 of those who were vaccinated at 57 days was undetectable, 100 and 1000, the corresponding vaccine protection was 50.8% (may be related to the effect of T cells), 90.7% and 96.1%.
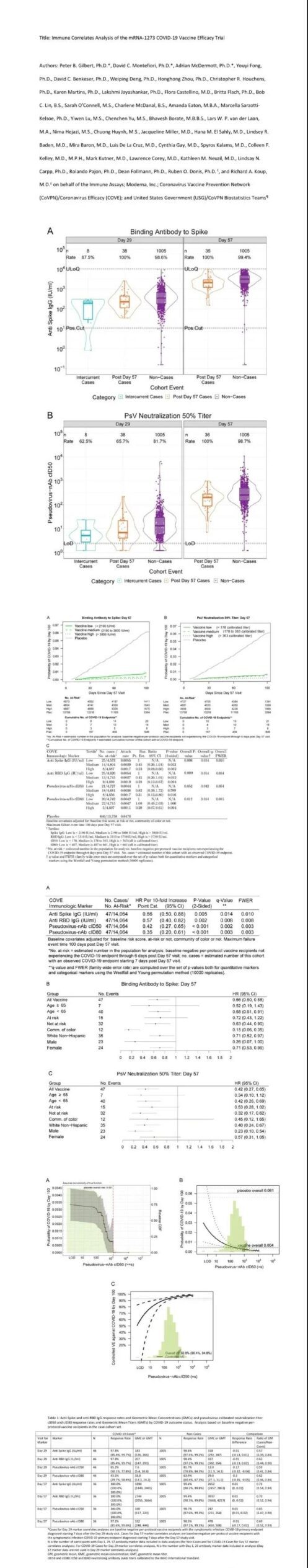
This study shows that the protective power of mRNA-1273 is largely based on inducing the production of neutralizing antibodies. Therefore, it is a very feasible method to predict the protective power of vaccines by neutralizing antibody titer.
Attached:
We have summarized some of the data on the neutralizing antibodies of the COVID-19 vaccine in the first phase, the protective power of the third phase in preventing infection with symptomatic COVID-19, and the changes in the neutralizing antibody after the third dose of intensive vaccination.
Image
(Early testing neutralizing antibody titers and fully testing the protection of vaccines against infection. Data collection and mapping: Hanson Clinical Research)
This paper not only confirms that the protective power of vaccines can be predicted by neutralizing antibody titers, it is also a very good research design template for other similar studies.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.