Her2 Breast Cancer: BRCA1 expression and radiotherapy sensitivity
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
Her2 Breast Cancer: BRCA1 expression and radiotherapy sensitivity
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
Her2 Breast Cancer: BRCA1 expression and radiotherapy sensitivity. Study on the relationship between the expression of HER2 and BRCA1 and the sensitivity of radiotherapy in breast cancer patients.
[Abstract]
Background and purpose: Breast cancer is the most common female malignant tumor in the world, and its incidence is still rising. Postoperative radiotherapy for breast cancer can effectively improve the local control rate and long-term survival rate. Radiotherapy has become the main technique for breast cancer treatment.
How to further reduce the adverse reactions of patients’ normal tissues and improve the effect of radiotherapy has become a research hotspot. To explore the expression of human epidermal growth factor receptor 2 (HER2) and breast cancer susceptibility gene 1 (BRCA1) in breast cancer patients, and study their expression and radiotherapy The relevance of sensitivity is expected to provide a basis for clinical treatment. Methods: The clinical data of cancerous tissues and adjacent normal breast tissues of 156 breast cancer patients admitted to the Affiliated Tumor Hospital of Zhengzhou University were reviewed and analyzed, and the benign breast lesions of 145 patients with benign breast lesions were selected as controls.
Real-time fluorescence quantitative polymerase chain reaction (RTFQ-PCR) was used to detect the expression of HER2 and BRCA1 mRNA in the tissue, and the level of HER2 and BRCA1 protein in the tissue was detected by immunohistochemistry. Spearman correlation analysis was used to analyze the relationship between HER2 and BRCA1 protein expression, Kaplan-Meier survival analysis was used to evaluate the survival rate of breast cancer patients after radiotherapy, and to explore the relationship between HER2 and BRCA1 expression and the prognosis of breast cancer patients with radiotherapy. Results: Compared with adjacent normal breast tissue and benign breast lesion tissue, the expression of HER2 mRNA and protein in cancerous tissue was up-regulated (P<0.01), and the expression of BRCA1 mRNA and protein was down (P<0.01).
After radiotherapy, the local failure rate of HER2-positive patients was significantly higher than that of negative patients (P<0.05), and the local failure rate of BRCA1-positive patients was significantly lower than that of negative patients (P<0.01). The 5-year survival rate of HER2-positive patients was significantly lower than that of HER2-negative patients (P<0.01), and the 5-year survival rate of BRCA1-positive patients was significantly higher than that of BRCA1-negative patients (P<0.01). Conclusion: Breast cancer patients with high expression of HER2 and low expression of BRCA1 gene are less sensitive to radiotherapy, which provides a new theoretical basis for the clinical treatment of breast cancer patients.
[Keywords] breast cancer; human epidermal growth factor receptor 2; breast cancer susceptibility gene 1; radiotherapy sensitivity; local failure rate
Breast cancer is one of the cancers with a higher mortality rate for women in the world [1]. The global incidence of breast cancer is increasing at an annual rate of about 2%. In recent years, the incidence and mortality of breast cancer in China are on the rise [2-3 ]. Radiotherapy is an important means of comprehensive treatment of breast cancer, and as a postoperative adjuvant treatment, it can reduce the local recurrence rate and reduce the risk of death [4].
The radiation resistance of breast cancer to radiotherapy is one of the main reasons that affect the curative effect [5-6]. Reducing the resistance of cancer to radiation and increasing the sensitivity of radiation are of great significance for improving the curative effect of breast cancer. Human epidermal growth factor receptor 2 (HER2) is a breast cancer proto-oncogene and the most representative breast cancer marker, breast cancer susceptibility gene 1 (BRCA1) It is a genetic predisposition to breast and ovarian cancer susceptibility gene, and is the first hereditary breast cancer susceptibility gene to be discovered [7-8]. HER2 is closely related to the development, malignant transformation and poor prognosis of breast cancer. Highly expressed HER2 is an important predictor of breast cancer targeted therapy effect and postoperative efficacy [9].
Research [10] found that 30% of breast cancer patients have high expression of HER2, which is an independent risk factor for poor prognosis. BRCA1 is a tumor suppressor protein that maintains genetic stability through transcription, cell cycle arrest and DNA repair [11]. BRCA1 affects the sensitivity of breast cancer cells to different chemotherapeutic drugs may have a two-way regulatory effect, which is closely related to the occurrence, development and prognosis of breast cancer.
In this study, real-time fluorescence quantitative polymerase chain reaction (RTFQ-PCR) and immunohistochemistry were used to detect HER2 and BRCA1 in cancerous tissues of breast cancer, adjacent normal breast tissues, and benign breast lesions. The changes in the expression of mRNA and protein are aimed to explore the relationship between the expression changes of the two and the prognosis of breast cancer radiotherapy, and provide relevant theoretical basis for the clinical treatment of breast cancer patients.
1. Materials and Method
1.1 Ethical review
The study was carried out voluntarily with the informed consent of the patients, and all patients had formulated a complete plan in advance and signed an informed consent form.
1.2 Research object
The clinical data of 156 breast cancer patients admitted to the Department of Breast Surgery of Zhengzhou University Cancer Hospital from May 2010 to May 2013 were selected for retrospective analysis. The average age was (45.6±5.5) years (35-63 years). Have received any radiotherapy, chemotherapy, endocrine therapy, and have not suffered from acute or chronic diseases or other malignant tumors in the past. Received modified radical surgery, postoperative adjuvant chemotherapy and radiotherapy (50 Gy/25 times, 5 times a week). Another 145 patients suspected to have breast cancer, but the pathological examination results showed that only patients with fibroids or benign breast lesions served as the control group. The average age was (46.2±5.4) years (32-58 years). Breast cancer patients and benign breast cancer patients There was no significant difference in the age and basic condition of patients with breast disease (P>0.05).
1.3 Specimen processing
After surgery, cancer tissues from breast cancer patients, normal breast tissues 2 cm from the cancer tissues, and benign breast lesion tissues were selected, and a part of the specimens were immediately stored in liquid nitrogen for RNA extraction. Another part of the specimens were soaked in 4% formaldehyde solution for 24 hours and preserved with paraffin-embedded sections for immunohistochemical detection.
1.4 RTFQ-PCR
Take out the sample tissue in liquid nitrogen, use TRIzol to extract RNA and determine its concentration and purity. According to the reverse transcription kit instructions, the RNA was reverse transcribed into 10 μL cDNA, and then the gene fragment was amplified by a PCR machine. The PCR conditions were: pretreatment at 95°C for 4 min, denaturation at 95°C for 30 s, annealing at 58°C for 5 s, and extension at 72°C for 5 s, a total of 30 cycles. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference, and the relative gene expression was calculated by the 2-ΔΔCt method. The RTFQ-PCR primers were synthesized by Shenggong Bioengineering (Shanghai) Co., Ltd. (Table 1).
1.5 Immunohistochemistry
The paraffin-embedded tissue specimens were sliced 4 μm serially and placed in gradient ethanol for dehydration for 15 min. Then the slices were immersed in hydrogen peroxide for inactivation treatment, washed with phosphate-buffered saline (PBS) 3 times, 5 min each time, and immersed in conventional goat serum for 15 min at room temperature. Add 20-30 μL of HER2 and BRCA1 antibodies diluted in PBS to the specimens, and incubate them overnight at 4°C. The negative control group uses PBS instead of the primary antibody. After incubation, wash with PBS 3 times, 5 min each time, and incubate for 45 min at 37°C with the secondary antibody dropwise. Then wash with PBS 3 times, 5 min each time, add diaminobenzidine (DAB) and stain for 5-10 min, rinse with tap water and counter-stain with hematoxylin for 5 min, and wash 5 min after desalting with dilute hydrochloric acid. min, then dehydrated, transparent, mounted, and observed under a microscope. The paraffin sections with positive expression of HER2 and BRCA1 proteins were used as the control group.
1.6 Dyeing result judgment
According to the results of immunohistochemical staining, yellow, brown or brown particles appearing in the tumor cell membrane or cytoplasm are defined as positive cells. Then randomly check 5 fields of view under a high-power microscope, the statistical results are: ①The percentage of statistically positive cells<10% is 0 points, 10%≤positive cell percentage<25% is 1 point, 25%≤positive cell percentage<50% 2 points, 3 points for the percentage of positive cells ≥50%; ②The statistical staining degree is 0 points for colorlessness, 1 point for yellow, 2 points for yellow-brown, and 3 points for tan. The two indicators are added together to form the final indicator: 0 to 1 is divided into “-“, 2 to 3 is divided into “+”, 4 to 5 is divided into “++”, and 6 is divided into “+++”.
1.7 evaluation criteria
If the tumor recurs in the chest wall or lymph node drainage area or part of the tumor tissue still exists 3 months after the end of radiotherapy, it is defined as a local failure. Combining computed tomography (CT) and pathological examination results to diagnose local failure, local failure rate = (number of locally uncontrolled patients) / (total number of patients) × 100%.
1.8 Patient follow-up
Collect clinical and histopathological data of all patients participating in the study, follow-up by telephone or collect clinical data, follow-up every 3 months for the first 3 years after treatment, and every 6 months for the next 3 to 5 years Follow-up until all the followed-up patients die, follow-up until May 2020.
1.9 Statistical processing
SPSS 22.0 software was used to analyze all the data. The data were expressed as x±s. The correlation coefficient was analyzed by Spearman correlation. Kaplan-Meier was used for survival analysis. P<0.05 was considered statistically significant.
2. Results
2.1 HER2 and BRCA1 mRNA expression in cancer tissue, benign breast lesion tissue and adjacent normal breast tissue
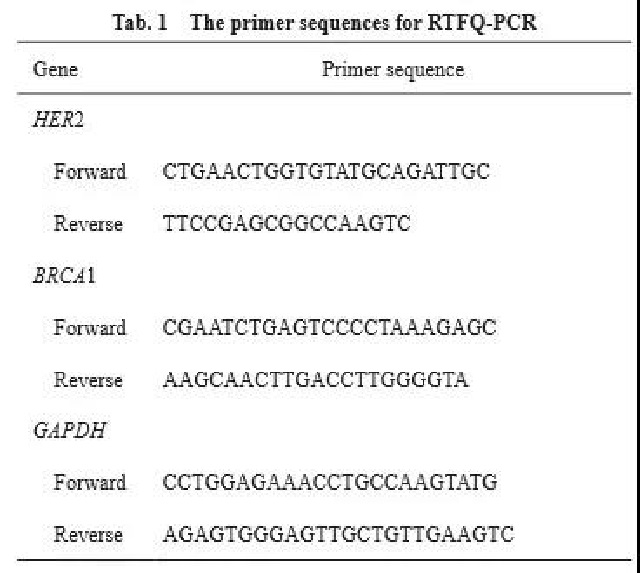
RTFQ-PCR results showed that there was no statistically significant difference in BRCA1 mRNA expression between benign breast lesions and adjacent normal breast tissues (P<0.05), and BRCA1 mRNA expression in cancer tissues was significantly lower than the other two groups (P<0.01, Figure 1A ). The expression of HER2 mRNA in benign breast lesions and adjacent normal breast tissues was not statistically significant (P<0.05), and the expression of HER2 mRNA in cancer tissues was significantly higher than that in the other two groups (P<0.01, Figure 1B). Compared with benign breast lesions and adjacent normal breast tissues, BRCA1 mRNA expression is low in cancer tissues, while HER2 mRNA expression is high.
2.2 HER2 and BRCA1 protein levels in cancer tissue, benign breast lesion tissue and adjacent normal breast tissue
The positive expression rate of HER2 in adjacent normal breast tissue, benign breast lesion tissue and cancer tissue were 11.5%, 18.5% and 49.4%, respectively. The positive expression rate of HER2 in cancer tissue was significantly higher (P<0.01, Figure 2A). The positive expression rates of BRCA1 in the three groups of samples were 66.7%, 55.9% and 32.7%, respectively. The positive expression rate of BRCA1 in cancer tissues was significantly reduced (P<0.01, Figure 2B), in benign breast lesions and adjacent normal breast tissues There was no statistically significant difference in the positive expression rate of HER2 and BRCA1 (P<0.05, Figure 2).
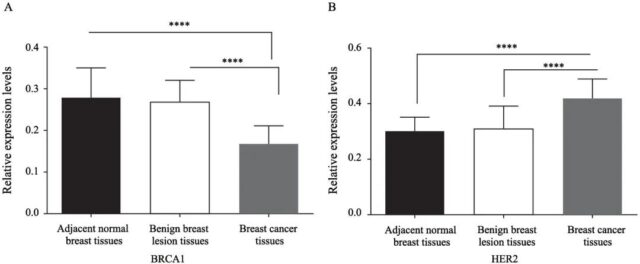
Figure 1 HER2 and BRCA1 mRNA expression in cancerous tissue, benign breast lesion tissue and adjacent normal breast tissue
Fig. 1 The mRNA expressions of HER2 and BRCA1 in breast cancer tissues, benign breast lesion tissues and adjacent normal breast tissues
A: The mRNA expression level of BRCA1 in each group; B: The mRNA expression level of HER2 in each group. ****: P<0.01, compared with benign breast lesion tissues and adjacent normal breast tissues
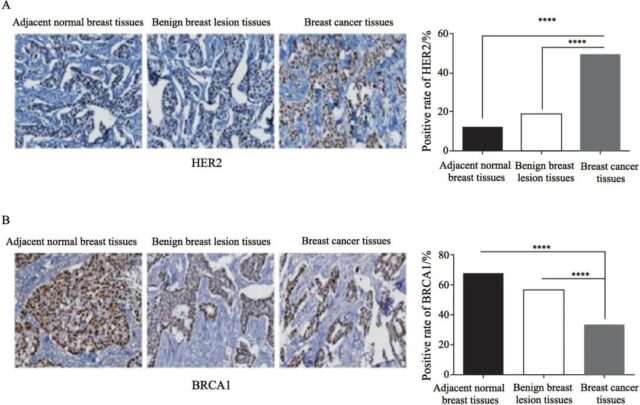
Figure 2 HER2 and BRCA1 mRNA expression in cancerous tissue, benign breast lesion tissue and adjacent normal breast tissue
Fig. 2 The mRNA expressions of HER2 and BRCA1 in breast cancer tissues, benign breast lesion tissues and adjacent normal breast tissues
A: The mRNA expression level of BRCA1 in each group; B: The mRNA expression level of HER2 in each group. ****: P<0.01, compared with benign breast lesion tissues and adjacent normal breast tissues
2.3 The relationship between the expression of HER2 and BRCA1 in cancer tissues and the sensitivity of radiotherapy
The local failure rate of HER2-positive patients was significantly higher than that of negative patients (P<0.05, Figure 3A); the local failure rate of BRCA1-positive patients was significantly lower than that of negative patients (P<0.01, Figure 3B).
2.4 The influence of different expressions of HER2 and BRCA1 on the prognosis of breast cancer patients
The 5-year survival rate of 156 breast cancer patients was 75.6%, and the survival time was 57 months. Among them, the 5-year survival rate of BRCA1-positive patients was 86.3% and that of negative patients was 70.5%. The difference was statistically significant (P=0.031, Figure 4A). The 5-year survival rate of HER2-positive patients was 67.5%, and that of negative patients was 83.5%, the difference was statistically significant (P=0.020, Figure 4B).
2.5 The correlation between HER2 and BRCA1 expression in breast cancer tissues
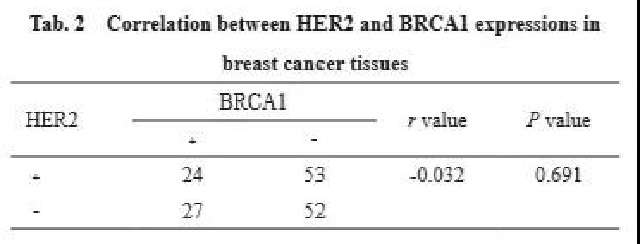
Among the 156 breast cancer tissue samples, 53 patients expressed HER2 positive expression and BRCA1 negative expression, 27 patients expressed HER2 negative expression and BRCA1 positive expression, 24 patients had positive expressions of both, and 52 patients had negative expressions of both (Table 2). The results of Spearman correlation analysis showed that there was no significant correlation between the expression of HER2 and BRCA1 (r=0.032, P=0.691).
Fig. 3 Relationship between HER2 and BRCA1 expressions in breast tissues and radiation sensitivity
A: Local failure rates in HER2-negative patients compared with HER2-positive patients after radiotherapy; B: Local failure rates in BRCA1-negative patients compared with BRCA1-positive patients after radiotherapy; *: P<0.05, compared with HER2-positive patients ; **: P<0.01, compared with BRCA1-positive patients
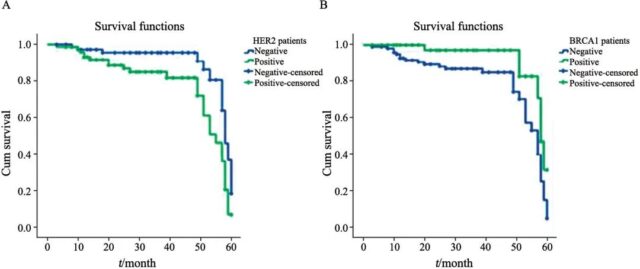 Fig. 4 The 5-year overall survival rates of patients with different expressions of HER2 and BRCA1 in breast cancer tissues
Fig. 4 The 5-year overall survival rates of patients with different expressions of HER2 and BRCA1 in breast cancer tissues
A: Comparison of 5-year survival rates between HER2-negative and HER2-positive breast cancer tissues; B: Comparison of 5-year survival rates between BRCA1-negative and BRCA1-positive breast cancer patients
3. Discussion:
HER2 is an important target for gastric cancer and breast cancer antibody therapy. At present, drugs targeting this target have been successfully used in clinical adjuvant therapy for patients. 20% to 25% of breast cancer patients have high expression of HER2 gene [12-13]. BRCA1 is a pleiotropic damage response protein, whose main function is DNA repair, target activation and protection of genomic double helix DNA from damage during DNA replication [14]. BRCA1 germline mutations are highly correlated with the radiosensitivity of ovary and breast cancer [15]. In this study, the mRNA expression and protein level of HER2 and BRCA1 in different tissues of breast cancer were detected, and the relationship between the expression of the two and the sensitivity of radiotherapy and the correlation with the prognostic survival time were analyzed, and the expression of the two and the expression of BRCA1 and the radiotherapy of breast cancer patients were explored. The relationship between sensitivity, with a view to provide a new clinical treatment method for breast cancer patients.
The high expression of HER2 will increase the survival rate, activity and proliferation rate of cancer cells, and play a certain inhibitory effect on many cancer treatment methods (such as endocrine therapy, radiotherapy, chemotherapy, etc.), and affect the therapeutic effect [16-17]. Tagliabue et al. [18] also confirmed that the high expression and activation of HER2 can lead to breast cancer. This study shows that compared with benign breast lesions and adjacent normal breast tissues, HER2 mRNA expression and protein levels in cancer tissues are increased, confirming that high HER2 expression is closely related to breast cancer lesions. BRCA1 is one of the important factors in the DNA repair system [19]. After DNA is damaged during cell division, BRCA1 will quickly phosphorylate to recognize the DNA damage signal or the signal of the S-phase DNA repair process. BRCA1 reduces the incidence of breast cancer. An important molecule that inhibits DNA damage has a significant inhibitory effect on the pathological process of breast cancer patients [20-21]. This study shows that BRCA1 mRNA expression and protein levels in breast cancer tissues are significantly reduced, and the reduction of BRCA1 expression levels can be used as one of the important monitoring indicators in the process of breast cancer carcinogenesis.
Research [22-23] reported that HER2 positive indicates a poor prognosis of breast cancer, and the survival rate of patients with negative expression of BRCA1 is lower than that of patients with positive expression of BRCA1. This study shows that the local failure rate of HER2-positive patients is higher than that of HER2-negative patients, and the local failure rate of BRCA1-positive patients is lower than that of BRCA1-negative patients. At the same time, this study also found that the 5-year survival rate of HER2-positive patients is lower than that of HER2-negative patients, and the 5-year survival rate of BRCA1-positive patients is higher than that of BRCA1-negative patients. It is speculated that the two may be important indicators of radiotherapy sensitivity and prognostic survival. Therefore, changing the expression levels of HER2 and BRCA1 in breast cancer may adjust the sensitivity of breast cancer patients to radiotherapy and affect the 5-year survival rate of patients.
To sum up, the local failure rate of HER2-positive patients in breast cancer tissues is high, and the prognosis is worse than that of HER2-negative patients, and the local failure rate of BRCA1-positive patients is lower, and the prognosis is significantly better than that of BRCA1-negative patients, suggesting the regulation of HER2 and BRCA1 Expression may have a certain promoting effect on improving the effect of radiotherapy in breast cancer patients. However, this study has problems such as limited sample size, incomplete index system, and incomplete index results, which need to be further studied.
Reference documents:
- ARNEDOS M, VICIER C, LOI S, et al. Precision medicine for metastatic breast cancer: limitations and solutions[J]. Nat Rev Clin Oncol, 2015, 12(12): 693-704.
- CEJALVO J M, PASCUAL T, FERNÁNDEZ-MARTÍNEZ A, et al. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer[J]. Cancer Treat Rev, 2018, 67: 63-70.
- QASEEM A, LIN J S, MUSTAFA R A, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians[J]. Ann Intern Med, 2019, 170(8): 547-560.
- BAGHSANGANI M K. Image thermography and computer aided diagnostic system and its usage in early breast cancer detection: a review[J]. Int Congr Ser, 2020. Online ahead of print.
- DATSENKO P V, GERASIMOV V A, BELIKOVA A A. Shortterm survival prediction scale in patients with metastatic brain disease caused by lung and breast cancer[J]. Zh Vopr Neirokhir Im N N Burdenko, 2020, 84(4): 26-35.
- YASUDA M T, SAKAKIBARA H, SHIMOI K. Estrogen- and stress-induced DNA damage in breast cancer and chemoprevention with dietary flavonoid[J]. Genes Environ, 2017, 39: 10.
- JANA SH, JHA BM, PATEL C, et al. CD10-a new prognostic stromal marker in breast carcinoma, its utility, limitations and role in breast cancer pathogenesis[J]. Indian J Pathol Microbiol, 2014, 57(4): 530 -536.
- IGNATOV T, EGGEMANN H, COSTA SD, et al. BRCA1 promoter methylation is a marker of better response to platinumtaxane-based therapy in sporadic epithelial ovarian cancer [J]. J Cancer Res Clin Oncol, 2014, 140(9): 1457- 1463.
- SWAIN S M, SCHNEEWEISS A, GIANNI L, et al. Incidence and management of diarrhea in patients with HER2-positive breast cancer treated with pertuzumab[J]. Ann Oncol, 2019, 30(8): 1404.
- TOMIGUCHI M, YAMAMOTO Y, YAMAMOTO-IBUSUKI M, et al. Fibroblast growth factor receptor-1 protein expression is associated with prognosis in estrogen receptor-positive/human epidermal growth factor receptor-2-negative primary breast cancer[J]. Cancer Sci , 2016, 107(4): 491-498.
- ZHANG W W, LUO J Y, YANG F, et al. BRCA1 inhibits AR-mediated proliferation of breast cancer cells through the activation of SIRT1[J]. Sci Rep, 2016, 6: 22034.
- SHAO XY, WANG X, XU XH, et al. Outcome prediction values of soluble human epidermal growth factor receptor-2 extracellular domain in metastatic breast cancer[J]. Int J Clin Exp Pathol, 2014, 7(3): 1108-1113 .
- IBRAHIM T, FAROLFI A, SCARPI E, et al. Hormonal receptor, human epidermal growth factor receptor-2, and Ki67 discordance between primary breast cancer and paired metastases: clinical impact[J]. Oncology, 2013, 84(3): 150157 .
- HOLLEBECQUE A, BAHLEDA R, FAIVRE L, et al. Phase I study of temsirolimus in combination with cetuximab in patients with advanced solid tumours[J]. Eur J Cancer, 2017, 81: 8189.
- EVRON E, AVRAHAM A, PALUCH-SHIMON S. Systemic treatment considerations for women with BRCA1/2-associated breast cancer[J]. Curr Breast Cancer Rep, 2014, 6(3): 139145.
- XU CS, CHEN HY, WANG X, et al. S100A14, a member of the EF-hand calcium-binding proteins, is overexpressed in breast cancer and acts as a modulator of HER2 signaling[J]. J Biol Chem, 2014, 289 (2): 827-837.
- KATCHMAN B A, ALAM R, FIELD M S, et al. Abstract 1318: a protein microarray signature of autoantibody biomarkers for the detection of HER2+ breast cancers[J]. Cancer Research, 2015, 75(15 Supplement):1318.
- TAGLIABUE E, REGONDI V, DE CECCO L, et al. Abstract P4-15-06: correlation between ERBB2 mRNA levels, HER2dependence and susceptibility to trastuzumab in human breast cancer[J]. Cancer Research, 2015, 75(9 Supplement): P415-06.
- SUN L, LIU J, WANG S, et al. Prevalence of BRCA1 gene mutation in breast cancer patients in Guangxi, China[J]. Int J Clin Exp Pathol, 2014, 7(9): 6262-6269.
- KIM D, JUNG W, KOO J S. The expression of ERCC1, RRM1, and BRCA1 in breast cancer according to the immunohistochemical phenotypes[J]. J Korean Med Sci, 2011, 26(3): 352-359.
- AHMED F, CHRISTOS PJ, SINGH P, et al. Analysis of outcomes in patients with BRCA1/2 breast cancer mutations treated with accelerated partial breast irradiation (APBI)[J]. Am J Clin Oncol, 2019, 42(5): 446 -453.
- WANG X Z, LIU H M, MAIMAITIAILI A, et al. Prevalence of BRCA1 and BRCA2 gene mutations in Chinese patients with high-risk breast cancer[J]. Mol Genet Genomic Med, 2019, 7(6): e677.
- ROBERT NJ, GOERTZ HP, CHOPRA P, et al. HER2-positive metastatic breast cancer patients receiving pertuzumab in a community oncology practice setting: treatment patterns and outcomes[J]. Drugs Real World Outcomes, 2017, 4(1): 1- 7.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



