The study subverted the perception of side effects of COVID-19 vaccine
- Did Cloud Seeding Unleash a Deluge in Dubai?
- Scientists Identify Gut Bacteria and Metabolites that Lower Diabetes Risk
- OpenAI’s Model Matches Doctors in Assessing Eye Conditions
- UK: A Smoke-Free Generation by Banning Sales to Those Born After 2009
- Deadly Mutation: A New Monkeypox Variant Emerges in the DRC
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
The study subverted the perception of side effects of COVID-19 vaccine
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The study subverted the perception of side effects of COVID-19 vaccine. NEJM: The largest real-world study in history, subverted the perception of the side effects of the COVID-19 vaccine.
The COVID-19 virus (SARS-CoV-2) COVID-19 pneumonia) caused (COVID-19) pandemic has passed more than a year, an unprecedented number of worldwide ongoing mass vaccination.
Phase 3 clinical trials have shown that several COVID-19 vaccines, including Pfizer/BioNTech’s BNT162b2 mRNA vaccine, have proven to be effective, but there are many potential risks of adverse events, including appendicitis, hypersensitivity, acute myocardial infarction and cerebrovascular accident.
However, because the number of participants is small and the sample population is healthier than average, a larger-scale safety assessment is needed.
In the past, people used passive monitoring systems to collect information on vaccine safety, which relied on voluntary reports by individuals who vaccinated, which would result in incomplete records.
The current active monitoring system can compare the target adverse event rate in the large electronic health record database with the background historical rate, which helps to highlight suspicious trends.
However, the lack of a strictly constructed control group in the active surveillance system restricts the ability to determine the causal relationship between vaccination and adverse events.
August 25, 2021, Israel’s Clalit Institute and Harvard University research team in the ” New England Journal of Medicine ” (NEJM) published a report entitled on: Safety at The BNT162b2 of Covid-19 mRNA Vaccine in Nationwide Setting A research paper .
The study used the comprehensive database of Israel’s largest healthcare organization to evaluate the safety of the BNT162b2 mRNA vaccine and found that the vaccine was not associated with the increased risk of most adverse events. In particular, the risk of developing myocarditis is actually higher among unvaccinated infected people .
After the new coronavirus infection, the risk of potentially serious adverse events such as myocarditis and many other serious adverse events is greatly increased, and the vaccine actually reduces these risks . This study shows that the BNT162b2 mRNA vaccine is very safe. These data are conducive to personal decision-making and dispel public doubts about the safety of the vaccine.

In order to provide the necessary background to interpret the vaccine safety results, this study examined for the first time various adverse events between vaccinated individuals and unvaccinated individuals infected with the coronavirus. Therefore, two separate analyses were performed: the vaccination cohort and the COVID-19 infection cohort analysis.
The vaccination cohort included 1,736,832 people , with a median age of 43 years. Subsequently, the research team carefully matched 884,828 vaccinated individuals 16 years and older with 884,828 unvaccinated individuals based on a wide range of social demographic, geographic, and health-related attributes. The median age of vaccinators was 38 years old, and 48% were women. The research team compared the incidence of 25 potential adverse events in the two groups within three weeks after vaccination. The analysis was carried out from December 20, 2020, the start of Israel’s national vaccination campaign to May 24, 2021.
Compared with the unvaccinated group, the risk of adverse events in the vaccinated group was significantly higher than that in the unvaccinated group: myocarditis occurred 2.7 times per 100,000 people in the vaccinated group, lymphadenopathy occurred 78.4 times per 100,000 people in the vaccinated group, and appendicitis occurred in 5.0 times per 100,000 people in the vaccinated group, and 15.8 times per 100,000 people in the vaccinated group. Vaccination has a significant protective effect on adverse events such as anemia, acute kidney injury, intracranial hemorrhage and lymphopenia.
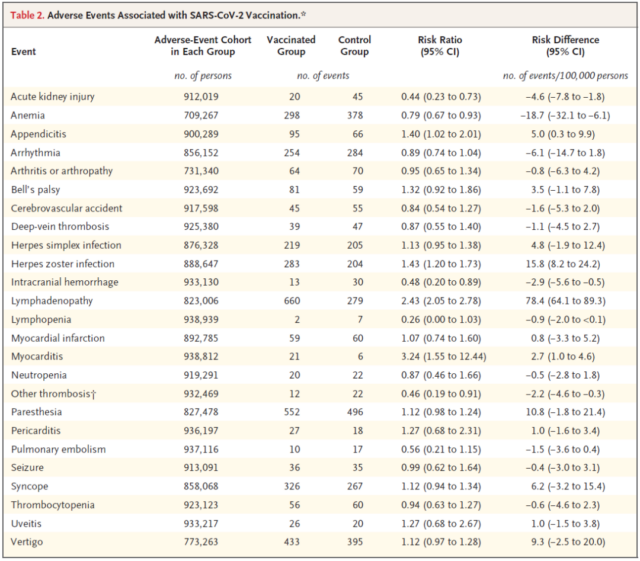
In order to provide the background of the above vaccine safety results, the research team conducted a separate analysis-the COVID-19 infection cohort analysis. A total of 233392 people (median age 36 years) were eligible for inclusion in the infection cohort. The analysis estimated the incidence of the same 25 potential adverse events among 173,106 unvaccinated individuals infected with the coronavirus, while 173,106 carefully matched controls were not infected with the coronavirus. The analysis is conducted from March 1, 2020 (the start of the COVID-19 pandemic in Israel) to May 24, 2021.
The research team found that myocarditis is related to the vaccine, but it is rare, 2.7 cases per 100,000 vaccinators. In contrast, among unvaccinated coronavirus-infected people, there are 11 cases of myocarditis per 100,000 infected people .
Other adverse events associated with vaccination are lymphadenopathy (inflammation or swelling of the lymph nodes) , which is a mild side effect that is part of the standard immune response to vaccination, and per 100,000 unvaccinated coronavirus infections. There are 78 cases among people, and among vaccinated people, there are only 5 cases for every 100,000 people.
Compared with the relatively small number of vaccine-related adverse reactions, the unvaccinated coronavirus infection has a high incidence of serious adverse events , including: arrhythmia (3.8 times higher than that of vaccinated persons, 166 per 100,000 infected persons Cases) , kidney injury (14.8 times increase; 125 cases per 100,000 infected persons) , pericarditis (5.4 times increase; 11 cases per 100,000 infected persons) , pulmonary embolism (12.1 times increase; per 100,000 infected persons) 62 cases) , deep vein thrombosis (increased by 3.8 times; 43 cases per 100,000 infected persons) , myocardial infarction (increased by 4.5 times; 25 cases per 100,000 infected persons), and stroke (increased by 2.1 times; present per 100,000 infected persons) 14 cases) .
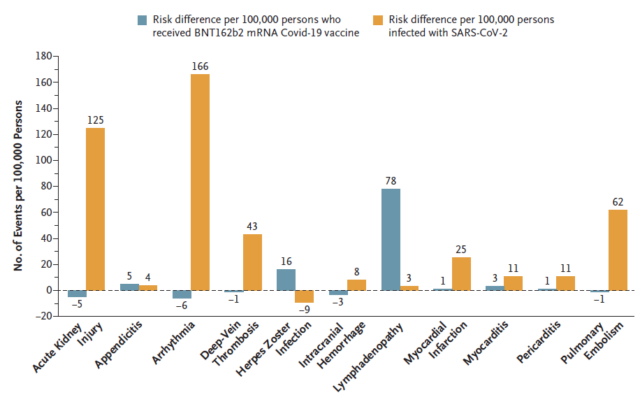
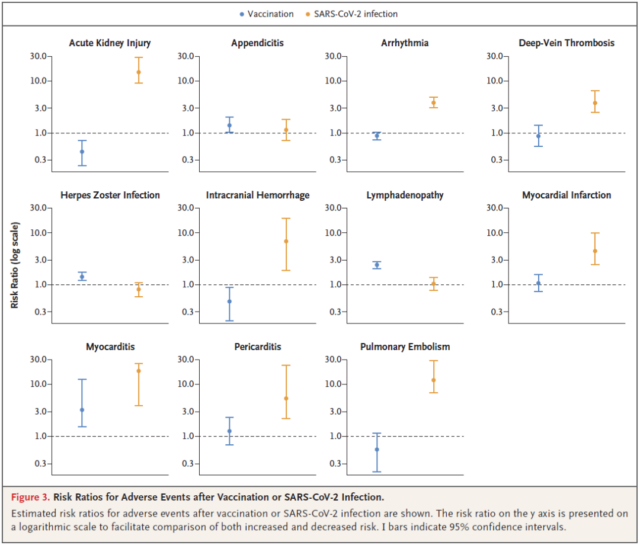
In short, the largest real-world research data on the side effects of the new coronavirus vaccine shows that the BNT162b2 mRNA vaccine has nothing to do with the increased risk of most adverse events, and serious risks such as myocarditis are actually higher in unvaccinated infected people. . This study shows that the BNT162b2 mRNA vaccine is very safe. These data are conducive to personal decision-making and dispel public doubts about the safety of the vaccine.
Paper link:
https://www.nejm.org/doi/10.1056/NEJMoa2110475
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?



