FIC: Sanofi Rilzabrutinib Pemphigus Phase 3 Clinical Failure
- Did Cloud Seeding Unleash a Deluge in Dubai?
- Scientists Identify Gut Bacteria and Metabolites that Lower Diabetes Risk
- OpenAI’s Model Matches Doctors in Assessing Eye Conditions
- UK: A Smoke-Free Generation by Banning Sales to Those Born After 2009
- Deadly Mutation: A New Monkeypox Variant Emerges in the DRC
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
Drug draft
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FIC: Sanofi Rilzabrutinib Pemphigus Phase 3 Clinical Failure
Rilzabrutinib is the result of Sanofi’s US$3.68 billion acquisition of PrincipiaBiopharma in August 2020. Rilzabrutinib is one of Principia’s two Phase 3 clinical phase products.
On September 9, the multinational pharmaceutical giant Sanofi announced that the Phase 3 clinical trial PEGASUS (NCT03762265) of its BTK inhibitor Rilzabrutinib for the treatment of pemphigus did not reach the primary and key secondary trial endpoints.

PEGASUS is a randomized, parallel grouped, double-blind, placebo-controlled Phase 3 clinical trial, and it is also the first placebo-controlled trial of BTK inhibitors in the treatment of pemphigus. 131 newly diagnosed or relapsed patients with moderate to severe pemphigus were recruited in 19 countries or regions around the world.
The primary endpoint was the complete response rate in patients who used the smallest dose (≤10 mg per day) of corticosteroids (CS) from week 29 to week 37. Complete remission is defined as the absence of new and established skin damage.
The key secondary endpoints include the cumulative CS dose (from baseline examination to the 37th week), the time to maintain complete remission with the minimum cumulative CS dose, and the time to complete remission for the first time with the minimum CS dose.
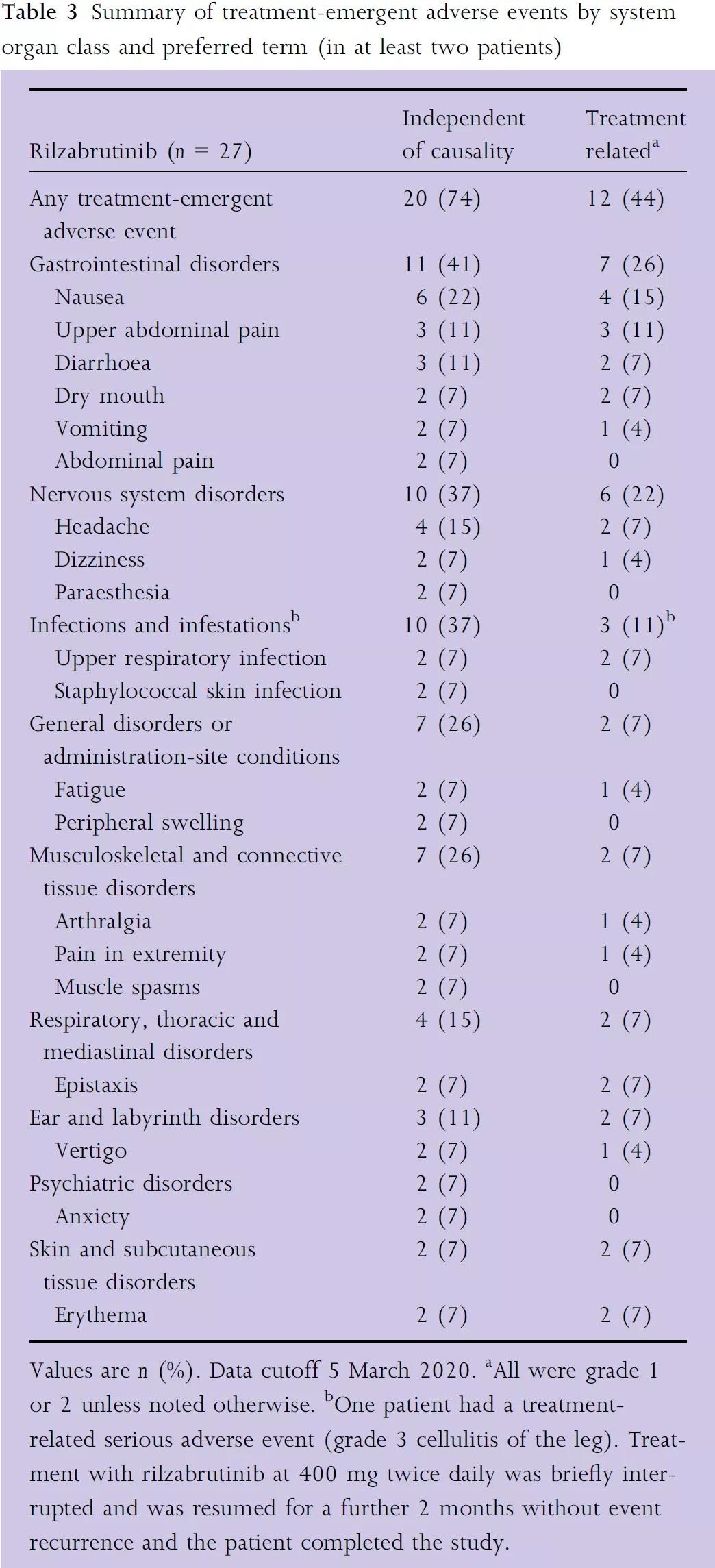
The security is the same as before.
In the phase 2 clinical trial BELIEVE (NCT02704429), although it was not compared with placebo, Rilzabrutinib performed quite well. The primary endpoint was reached: the proportion of patients who received zero-dose to low-dose (≤0.5 mg per day) CS treatment maintained disease activity control (no new lesions or healing of existing lesions, CDA) within 4 weeks, and the safety was acceptable.
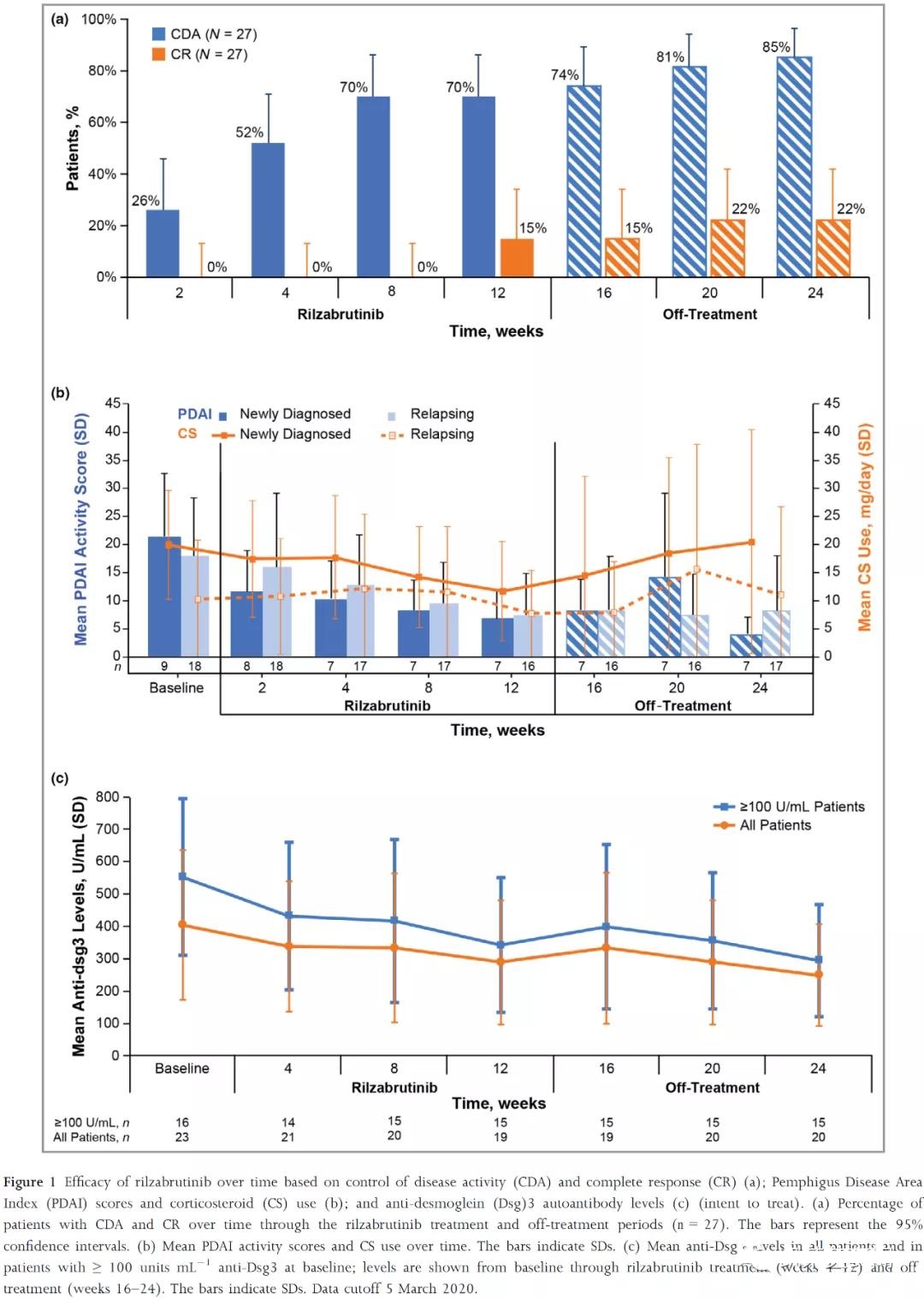
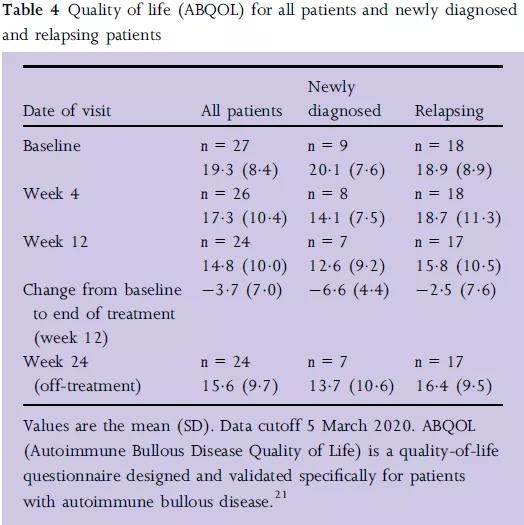
At the same time, the quality of life of patients is significantly improved.
Rilzabrutinib is the result of Sanofi’s US$3.68 billion acquisition of PrincipiaBiopharma in August 2020. Rilzabrutinib is one of Principia’s two Phase 3 clinical phase products.
In addition to the indications for pemphigus, Rilzabrutinib’s development indications also include immune thrombocytopenia (ITP), IgG4-related diseases (IgG4-RD), etc., and has been granted by the FDA to treat immune thrombocytopenia (ITP) rapid Channel Qualification (FTD) and Orphan Drug Qualification (ODD).
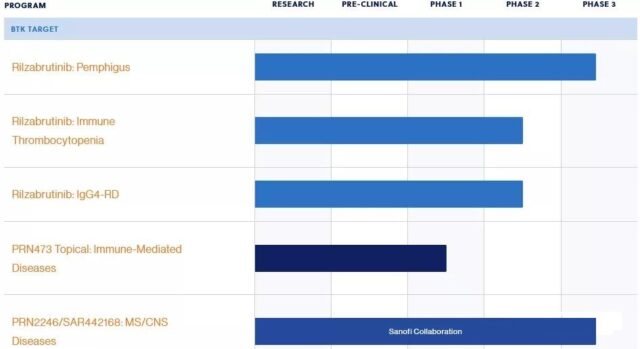
Pemphigus is a life-threatening immune-mediated disease characterized by blisters on the mucous membranes and skin. Currently, treatment options for pemphigus (including pemphigus vulgaris and pemphigus phyllodes) are limited, and systemic corticosteroid therapy is still the standard treatment. Sanofi will continue to evaluate the Phase 3 clinical data and plan to share detailed results in future medical conferences.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



