What are the drugs of targeted therapy for T cell lymphoma?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
What are the drugs of targeted therapy for T cell lymphoma?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What are the drugs of targeted therapy for T cell lymphoma?
T-cell lymphoma is a rare non-Hodgkin’s lymphoma, which can be roughly divided into peripheral T-cell lymphoma and cutaneous T-cell lymphoma.
At present, the first-line treatment of peripheral T-cell lymphoma often adopts CHOP or CHOP-like chemotherapy, while the treatment of cutaneous T-cell lymphoma is mainly divided into systemic treatment and local treatment.
However, T-cell lymphoma has a poor prognosis, poor sensitivity to chemotherapy, and a low overall patient survival rate.
The research progress of molecular genetics and the in-depth exploration of disease mechanisms have promoted the development of new treatment options and targeted drugs, and provided new treatment options for the treatment of T-cell lymphoma.
This article reviews the targeted therapy drugs for T-cell lymphoma.
T-cell lymphoma is a rare type of non-Hodgkin’s lymphoma (NHL) that originates from mature T lymphocytes and can be roughly divided into peripheral T-cell lymphomas (PTCL) and skin T-cell lymphomas Tumor (Cutaneous T-cell lymphomas, CTCL).
PTCL accounts for about 5% to 10% of all NHLs in Western populations, and 15% to 20% of all NHLs in Asian populations.
In the 2017 WHO classification of hematopoietic and lymphatic system tumors, PTCL is divided into 23 well-defined subtypes and 6 tentative subtypes. Among them, PTCL not otherwise specified (PTCL-NOS) and vascular immunoblasts T-cell lymphoma (Angioimmunoblastic T-cell lymphoma, AITL) is the most common, other subtypes also include: extranodal NK/T-cell lymphoma (ENKTL) nasal type, adult T-cell leukemia/lymphoma (ATL), anaplastic lymphoma Oncokinase (ALK) positive anaplastic large cell lymphoma (ALCL), ALK negative anaplastic large cell lymphoma, etc.
CTCL is the most common type of skin lymphoma, usually clinically presented as red, scaly plaques or thickened skin plaques, similar to eczema or chronic dermatitis.
Among the subtypes of CTCL, Mycosis fungoides (MF) is the most common subtype of CTCL.
- Characterized by the clonal proliferation of effector memory T cells resident in the skin;
- Sézary syndrome (Sézary syndrome, SS) is a type of leukemia characterized by the clonal proliferation of central memory T cells;
- Other subtypes include lymphomatoid papulosis (Lymphomatoid papulosis, Lyp),
- Primary cutaneous anaplastic large cell lymphoma (Primary cutaneous anaplastic large cell lymphoma, pcALCL)
- Other rare primary skin peripheral T-cell lymphomas, etc.
At present, the first-line treatment of PTCL usually adopts CHOP (Cyclophosphamide, Doxorubicin, Vincristine and Prednisone) or CHOP-like regimens (Cyclophosphamide, Vindesine) , Epirubicin, prednisone and etoposide) chemotherapy.
As for the treatment of CTCL, it is mainly divided into system treatment and local treatment. However, T-cell lymphoma has a poor prognosis, poor sensitivity to chemotherapy, and a low overall patient survival rate.
With the development of molecular genetics and the in-depth understanding of disease mechanisms, new treatment options and targeted drugs continue to appear, providing new treatment options for the treatment of T-cell lymphoma.
Targeted therapy drugs
With the understanding of various key pathways in the generation of T-cell lymphoma and the identification of therapeutic targets, more targeted therapy strategies have emerged. For example, biomarker-driven PTCL targeted therapy strategies involve targeting cell surface receptors. Body, targeting epigenome, targeting tumor microenvironment, and targeting signal transduction and proliferation pathways, etc. (Table 1).
Since the US FDA approved Rituximab (Rituximab) for relapsed or refractory B-cell lymphoma in 1997, the development and approval of new targeted therapies for B-cell lymphoma has surged.
As for T-cell lymphoma, a considerable amount of basic research and translational research has been completed, and many new targeted therapies have been confirmed for their efficacy, which has greatly promoted the development of new drugs, including new antibody therapeutics, small molecule inhibitors, and immune systems. T-cell lymphoma targeted therapy drugs such as therapy and immunomodulators (Figure 1 and Table 2).
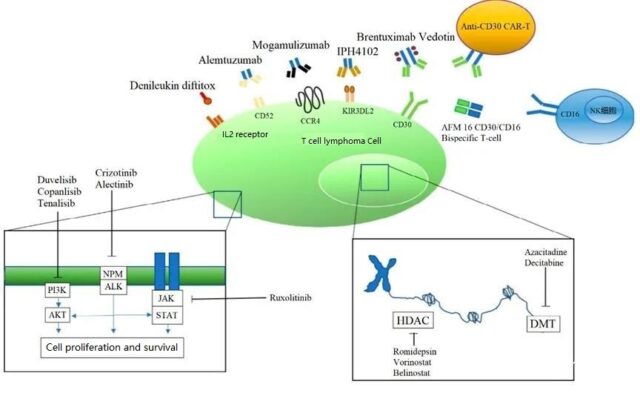
Note: PI3K: Phophoinositide3-kinase; NPM: Nucleophosmin; ALK: Anaplastic lymphoma kinase; JAK: Janus kinases; STAT: Signal transducer and activator of transcription proteins; HDAC: Histone deacetylase; DMT: DNA methyltransferase.
Figure 1: Targets of new T-cell lymphoma therapeutic agents
Among them, monoclonal antibody drugs have the advantages of target specificity, accurate killing of tumor cells, and relatively few adverse reactions. They have shown curative effects in some T-cell lymphoma subtypes; and for small molecule inhibitors of targeted signaling pathways, It is possible to detect changes in signal pathway-related genes or protein levels based on clinical studies, and to screen out patients sensitive to these drugs for better treatment. However, the experience of small molecule inhibitors is still relatively limited, and more clinical studies are still needed Combined trials of data and other drugs further support its effectiveness; in terms of immunotherapy and immunomodulators, relevant clinical trials are currently ongoing. Below, the representative drugs in each category are introduced.
BV
BV (Adcetris, Verbutuximab) is a conjugate of anti-CD30 monoclonal antibody and anti-tubulin monomethyl Auristatin E (MMAE), which can make use of the endocytosis of the cell itself to allow small chemical molecules to enter tumor cells The drug effect occurs inside, so as to achieve the purpose of killing tumor cells.
Currently, BV has been approved for the treatment of T-cell lymphoma in many countries. For example, in 2017, the US FDA approved BV to treat adult patients with pcALCL and CD30-expressing MF who had received systemic therapy.
In 2018, the FDA approved the BV combined chemotherapy regimen CHP (cyclophosphamide + adriamycin + prednisone) for the first-line treatment of systemic anaplastic large cell lymphoma (sALCL) or other CD30-positive PTCL (including AITL and unspecified PTCL) adult patients.
In May 2020, the European Commission (EC) approved the expansion of BV’s conditional marketing authorization to include: combined chemotherapy regimen CHP, first-line treatment of sALCL adult patients with tumors expressing CD30 (CD30 positive); this approval applies to all EU member states, In Norway, Liechtenstein, and Iceland, it is worth mentioning that BV is the first and only targeted drug approved for the first-line treatment of sALCL in decades. Immediately afterwards, the National Medical Products Administration (NMPA) of China also approved BV for the treatment of adult patients with relapsed or refractory sALCL or CD30-positive Hodgkin lymphoma (HL).
Mogamulizumab
Mogamulizumab (Poteligeo) is a humanized monoclonal antibody targeting CC chemokine receptor 4 (CCR4) developed by Kyowa Fermentation Kirin. The sugar chain of its Fc segment has been modified by demethoxylation to enhance effector cells. Fcγ receptor binding capacity, resulting in higher antibody-dependent cell-mediated cytotoxicity activity.
CCR4 is highly expressed in type 2 helper T cells and regulatory T cells, and is also highly expressed in tumor cells of some PTCL subtypes such as ATL.
In 2018, the FDA approved the marketing of Mogamulizumab to treat adult patients with relapsed or refractory MF or SS who have received at least one systemic therapy. This is the first time the FDA has approved a drug for SS, and it also provides more treatment options for MF patients.
Denileukin diftitox
Denileukin diftitox is a fusion protein of interleukin-2 (IL-2) receptor binding site and diphtheria toxin, which specifically binds to the IL-2 receptor on the surface of tumor lymphocytes, and then delivers and releases diphtheria into the cell toxin.
The anti-tumor effect of the drug relies on the intracellular delivery of diphtheria toxin, which can inhibit protein synthesis and induce cell death.
Denileukin diftitox received accelerated approval in the United States in 1999 under the brand name Ontak for the treatment of persistent or recurrent CTCL. In 2020, Eisai submitted a marketing authorization application (MAA) for the anticancer agent Denileukin diftitox in Japan for the treatment of relapsed or refractory CTCL and PTCL.
In May 2021, Eisai has launched Remitoro (Denileukin diftitox) intravenous infusion 300μg dosage form in Japan for the treatment of relapsed or refractory PTCL and relapsed or refractory CTCL. According to a survey conducted by the Japanese Ministry of Health, Labour and Welfare (MHLW), it is estimated that there are less than 6,000 PTCL patients and less than 4,000 CTCL patients in Japan. The prognosis and treatment of these patients are not optimistic.
Nivolumab
PD-L1 (Programmed death ligand 1) is overexpressed in the microenvironment of T-cell lymphoma and is a reasonable therapeutic target.
Due to the efficacy of PD1 (Programmed death 1) and PD-L1 inhibitors in solid cancer, they have been rapidly developed as a class of drugs and have been tested in a series of hematological malignancies.
Nivolumab (Opdivo) is a PD-1 immune checkpoint inhibitor that can bind to the PD-1 receptor expressed on activated T cells and block the binding of PD-1 to PD-L1/PD-L2, thereby deactivating immunity The PD-1 pathway in the system is an inhibitory signal to the immune system, and one of the manifestations of the inhibitory signal is to interfere with the anti-tumor response.
In the phase I trial of Nivolumab in the treatment of patients with hematological malignancies, 13 cases of MF, 5 cases of PTCL and 5 cases of “other” T-cell lymphoma patients were included. Among them, the research data showed that the ORR was 17%, and no patients got CR, but due to concerns about excessively progressive disease, the subsequent phase II trial was stopped early.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



