Low-dose radiotherapy reverses immunotherapy resistance
- Engineered Soybeans with Pig Protein: A Promising Alternative or Pandora’s Dish?
- Severe Fever with Thrombocytopenia Syndrome (SFTS): A Tick-Borne Threat with High Mortality
- Why Isolating Bananas Extends Their Shelf Life?
- This common vitamin benefits the brain and prevents cognitive decline
- New report reveals Nestlé adding sugar to infant formula sold in poor countries
- Did Cloud Seeding Unleash a Deluge in Dubai?
Cancer Discovery: Low-dose radiotherapy reverses immunotherapy resistance & tumor immune desertification!
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Cancer Discovery: Low-dose radiotherapy reverses immunotherapy resistance & tumor immune desertification!
Magazines and impact factors:

Cancer Discovery: Low-dose radiotherapy reverses immunotherapy resistance & tumor immune desertification!
Significance:
There are many studies on tumor immunophenotyping. Among them, the three major immunophenotypes of tumors are widely recognized by researchers: “immune infiltrating type”, “immune rejection type”, and “immune desert type”. “Immune desert type” tumors rarely infiltrate CD8+ T cells, and the tumor immune response is poor. Low-dose radiotherapy (LDRT) combined with immune checkpoint inhibitors (ICB) can effectively control tumor metastasis by mobilizing the participation of innate immunity and adaptive immunity, and down-regulating the immunosuppressive factor TGFb.
However, whether and what role ICB plays in the treatment of ovarian cancer is still unknown. This study found that the combination of LDRT and reasonable immunotherapy has a novel and important synergy in the treatment of immune-infiltrating tumors. The key is to activate multiple innate and adaptive immune pathways at the same time, revealing the relationship between LDRT and immune regulation. The interdependence, which leads to a strong anti-tumor immune mobilization.
Guide
At present, although the response of some patients to ICB is stable and long-lasting, there are still a considerable number of cancer patients who have a low response to ICB. Therefore, it is particularly important to determine an effective and feasible plan for these patients. In addition, the inherent plasticity of the tumor and its microenvironment makes it possible to dynamically up-regulate multiple inhibitory mechanisms. Therefore, different combinations of therapies are required to maintain the control of the tumor by T cells.
Previous research data showed that in a mouse local neuroendocrine pancreatic tumor model, low-dose radiotherapy (LDRT) can reorganize the tumor microenvironment, induce the polarization of macrophages M1, and M1 macrophages can produce related Chemokines recruit effector T cells and at the same time induce tumor blood vessel normalization. LDRT combined with ICB therapy can effectively control tumor metastasis through the participation of innate immunity and adaptive immunity, as well as down-regulation of the immunosuppressive factor TGFb. However, whether and what role ICB plays in the treatment of ovarian cancer is still unknown.
Recently, the research team of the Ludwig Institute for Cancer Research at the University of Lausanne published an online article titled Low Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy in the Cancer Discovery journal. Through exploration, the researchers confirmed a reasonable combination of treatments. Through the simultaneous mobilization of innate immunity and adaptive immunity, immune checkpoints on effector T cells, regulatory T cells (Treg) and antigen-presenting cells (APCs) can be solved simultaneously to control tumors.
 Image source: Cancer Discovery
Image source: Cancer Discovery
Main research content
Low-dose total abdominal radiotherapy induces immune cell infiltration in advanced orthotopic ovarian cancer
In order to evaluate the effect of low-dose total abdominal radiotherapy (LD-WART) on ovarian cancer, they chose an orthotopic abdominal cavity ID8 mouse model and found that LD-WART is sufficient to induce important transcriptional changes in immune cells in the body, especially inflammation. Significant up-regulation, including IFNa and IFNg responses, activation of complement, up-regulation of interleukin 6 / JAK / STAT3 signaling, expression of key chemokines that attract T cells and NK cells, and other inflammatory markers.
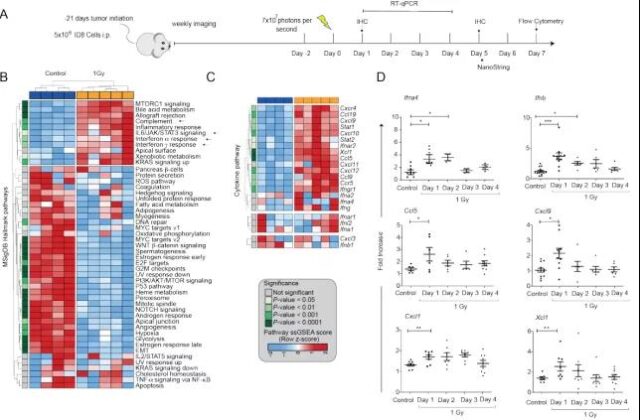
Image source: Cancer Discovery
Low-dose radiotherapy enhances tumor response to immunotherapy
Next, the researchers tried to develop a combination therapy strategy to address the immune targets upregulated by LDRT: low-dose cyclophosphamide treatment on day 0, LD-WART, ICB, and aCD40 antibody (called radiation) on day 1. Combination immunotherapy, Radio-combinatorial immunotherapy, RACIM), 3 times a week. Surprisingly, 83.5% of the mice that received RACIM showed a response on the 20th day of treatment, with 14% in complete remission and 11% in partial remission. However, most partially responding mice progressed after stopping treatment, with a median overall survival of 69 days.
On the 90th day, all surviving mice were disease-free through imaging and pathological examinations, and the total cure rate was 15%. It is worth noting that the combined immunotherapy group (low-dose cyclophosphamide + ICB + aCD40 antibody, CIM group) lacking LDRT did not show a therapeutic effect. At the genetic level, compared with CIM or untreated control tumors, after RACIM treatment, genes related to T cells, monocytes, and DCs were significantly up-regulated.
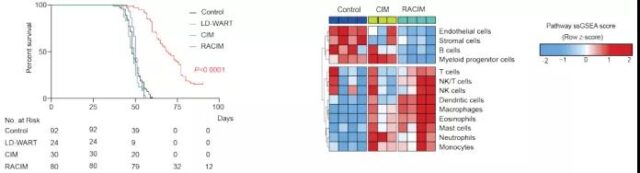 Image source: Cancer Discovery
Image source: Cancer Discovery
RACIM increases the number of tumor suppressor CD4+ and CD8+ T cells
Subsequently, in order to explore whether the efficacy of RACIM is further related to the difference in the quality of tumor infiltrating T cells (TILs), they used scRNAseq to analyze TILs 5 days after the second LDRT cycle. The results of the analysis identified a total of 9 different T cell states. TILs treated with RACIM are highly enriched in the activated effector T cell population; in addition, compared with the tumors treated with CIM, the expansion of CD4+ depleted T cells after RACIM treatment has the largest difference. At the same time, compared with CIM, the expression of Ifng, Prf1 and Gzmb of CD4+ depleted T cells was significantly increased after RACIM treatment, and these genes are related to effector function and cytolytic ability. The above findings indicate that RACIM therapy recruits a large number of low-clonal CD4+ cells into the tumor. These cells acquire the characteristics of exhaustion, but also exhibit important effector functions.
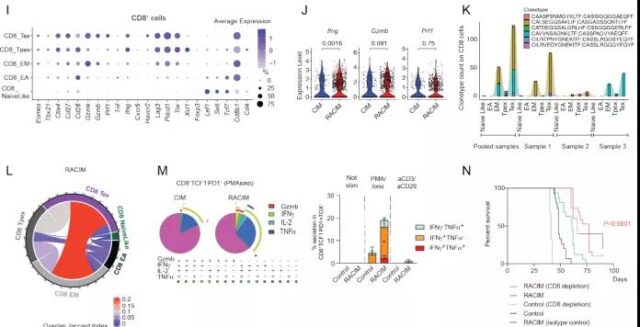 Image source: Cancer Discovery
Image source: Cancer Discovery
Compared with CIM, after RACIM treatment, the CD8+ TIL population showed higher exhaustion, costimulation, and effector functions. Similar to CD4+, RACIM-treated tumors have the highest clonal expansion ratio of CD8+ T cells, and CD8+ depleted T cells have the highest clonal expansion ratio.
Therefore, these results indicate that LDRT has a significant ability to resist tumors by recruiting T cells, especially by expanding the number of activated, functionally effective CD4+ and CD8+ T cells, in combination with immunotherapy.
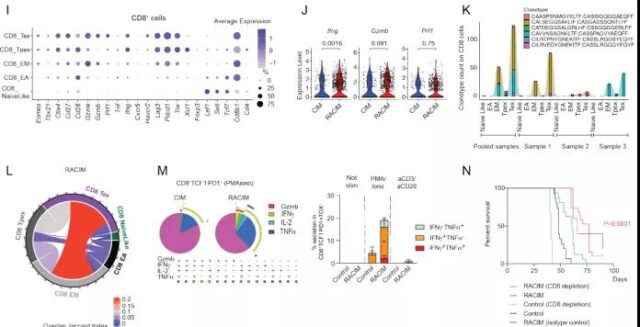
Image source: Cancer Discovery
Next they examined the effect of the combination therapy on myeloid cells. Through single-cell transcriptome sequencing analysis of CD11b+ cells from untreated, CIM- or RACIM-treated tumors, a total of 29 myeloid cell transcriptome states were found, which were divided into three main groups according to the main characteristic genes: macrophages , DCs and monocytes. RACIM drives important reprogramming of the tumor myeloid system relative to CIM therapy, inducing profound changes in all three myeloid groups.

Image source: Cancer Discovery
Low-dose radiotherapy combined with ICB induces human advanced tumor response
Subsequently, the researchers shifted the focus of the study to the patients. They used the same dose of CIM for all patients but different doses of radiotherapy: the first 3 patients received 0.5 Gy treatment in each lesion, and the other 5 patients received treatment at each lesion. The lesion received 1 Gy treatment. A total of 3 patients (37.5%) were observed to have reduced tumor size in the targeted irradiated lesions, the other 4 patients were in stable condition, the overall disease control rate was 87.5%, and 1 patient (12.5%) was diagnosed with disease progression.
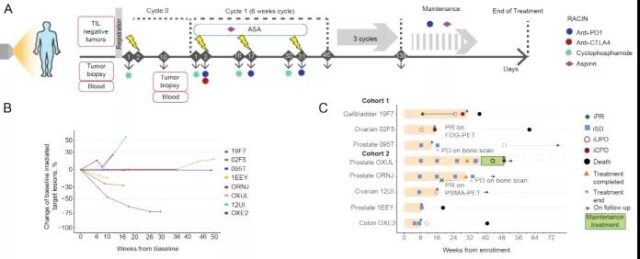
Image source: Cancer Discovery
So is the biological effect of LDRT in human tumors similar to that of mouse tumor models? They analyzed the biopsy results of the same metastases at baseline and 7-10 days after the first LDRT and before the start of ICB. It was found that there was a consistent tumor reduction after combined LDRT immunotherapy.
In addition, similar to the mouse model, they observed significant T cell infiltration in responding tumors, which were mainly composed of CD4+ cells; analysis of differential gene expression in biopsies before and after irradiation showed that responding tumors and non-responsive tumors The response mode of LDRT is different. In addition, responsive tumors subsequently showed a significant increase in Th1 signaling, while non-responsive tumors were characterized by an increase in M2 macrophages.
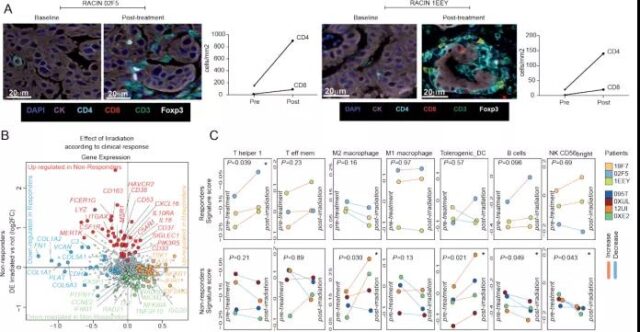
Image source: Cancer Discovery
Therefore, just like in mice, LDRT successfully resets the immune microenvironment of tumor patients, that is, recruits innate and adaptive immune cells. In fact, there is also an important overlap in gene markers that respond to mouse and human tumors, and Th1 markers have reached statistical significance in both. Finally, using spatial transcription profiling, they tested whether the intraepithelial tumor cavity is involved in response to immune activation in tumors. It was found that after LDRT, responding tumors showed an increase in Th1, CD8+ and TEM markers mainly located in the islets of the tumor; on the contrary, non-responsive tumors showed up-regulation of M2 macrophages and neutrophil signals after LDRT treatment. Mainly detected in the tumor stroma.
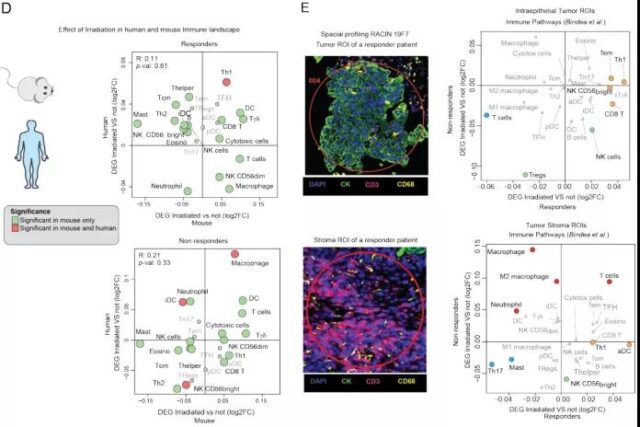
Image source: Cancer Discovery
Concluding remarks
In summary, this study proved a novel and important synergy of LDRT and reasonable immunotherapy in the treatment of immune-infiltrating tumors. The key is to activate multiple innate and adaptive immune pathways at the same time, revealing LDRT The interdependence between immune regulation and immune regulation has led to powerful anti-tumor immune mobilization.
More importantly, their preliminary clinical data indicate the importance of radiotherapy for all metastatic tumors, because in responding patients, a long-lasting response was only observed in the radiation lesions, which was initially considered non-pathological The sexual lesions (and therefore not the area of radiotherapy) eventually turned out to be progressive metastatic deposits. Future research should focus on improving combination strategies to further enhance this synergy and make it produce important protective memories.
Article link: https://cancerdiscovery.aacrjournals.org/content/early/2021/09/03/2159-8290.CD-21-0003
Cancer Discovery: Low-dose radiotherapy reverses immunotherapy resistance & tumor immune desertification!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



