What are new developments of CDK4/6 | PI3K inhibitors for breast cancer?
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
- Kochi University pioneers outpatient bladder cancer treatment using semiconductor lasers
- ASPEN 2024: Nutritional Therapy Strategies for Cancer and Critically Ill Patients
- Which lung cancer patients can benefit from neoadjuvant immunotherapy?
- Heme Iron Absorption: Why Meat Matters for Women’s Iron Needs
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
ESMO 2021: What are new developments of CDK4/6 | PI3K inhibitors for breast cancer?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What are new developments of CDK4/6 | PI3K inhibitors for breast cancer?
ESMO 2021 | What are the new developments of CDK4/6 and PI3K inhibitors in the field of breast cancer treatment? These 4 clinical studies must be watched!
POSEIDON randomized phase II trial: Tamoxifen (TAM) + taselisib or placebo (PLA) in the treatment of HR+/HER2- metastatic breast cancer (MBC) patients
Abstract LBA18
Background
Taselisib is an oral inhibitor of PI3K’s class I α, δ, and ϒ subtypes and has been shown to be clinically active when used in combination with TAM (Baird R et al., CCR 2019).
Methods
POSEIDON is an international, multi-center, randomized (1:1) phase II trial for TAM + taselisib or PLA in HR+/HER2- MBC patients after previous endocrine therapy (ET). Allow to cross.
Primary endpoint: unstratified progression-free survival (PFS; local assessment). Secondary endpoints: safety, RECIST 1.1 overall response rate (ORR; complete response [CR] + partial response [PR]), clinical benefit rate (CBR; CR + PR + stable disease> 6 months) and overall survival (OS). A constant hazard ratio (HR) of 0.64 (β=90%, α=0.2 on both sides) for PFS requires 180 patients.
Results:
152 patients (median age 63 years) were enrolled in the group (table), and the median follow-up was 26.4 months (m). After adding taselisib to TAM, the median PFS increased from 3.2 m to 4.8 m (unstratified HR = 0.62, 95% CI: 0.43-0.93, p = 0.02; stratified HR=0.68, 95% CI: 0.4-1.2 , P = 0.16), has nothing to do with CA status.
In the taselisib group, ORR=11.8% (95%CI: 5.6-21.3) and CBR=22.4% (95%CI: 13.6-33.4); in the PLA arm, ORR=2.6% (95%CI: 0.3-9.2 ) And CBR=14.5% (95%CI: 7.5-24.4).
Reasons for stopping taselisib / PLA: toxicity 22% / 4%, disease progression 55% / 67%, other (mainly Covid-19) 23% / 29%. Common adverse events (AE) in the Taselisib group: diarrhea (36%), nausea (35%), hyperglycemia (28%). Common AEs in the PLA arm: nausea (21%), fatigue (16%). G3-5 AEs with Taselisib are more common (44% vs 5%, p<0.01), mainly diarrhea (11%), hyperglycemia (5%) and transaminase (5%).
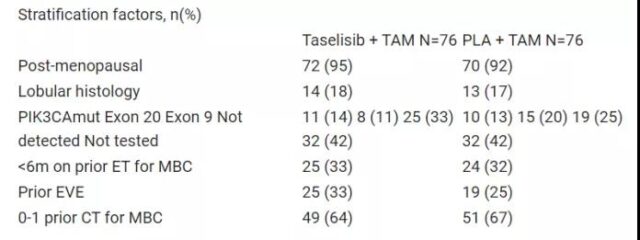
Conclusion
Adding Taselisib to TAM can increase PFS in patients with HR+/HER2-MBC, but this regimen is poorly tolerated. Combining drugs with better therapeutic index to inhibit the ET and PI3K-AKT pathways requires additional research in the subgroup of breast cancers most likely to benefit.
Clinical trial identification: EudraCT 2013-003947-51; NCT02301988.
Oliveira M, et al. POSEIDON randomized phase II trial: Tamoxifen (TAM) + taselisib or placebo (PLA) in patients (pts) with hormone receptor positive (HR+)/HER2- metastatic breast cancer (MBC). Annals of Oncology (2021 ) 32 (suppl_5): S1283-S1346. 10.1016/annonc/annonc741.
PALOMA-4: Efficacy of pabociclib + letrozole (LET) and placebo (PBO) + LET in ER+/HER2- advanced breast cancer (ABC)
Background
In the past 40 years, the incidence of breast cancer among Asian women has increased rapidly. A previous subgroup analysis from PALOMA-2 indicated that PAL + LET may be effective as a first-line treatment for postmenopausal Asian women with ER+/HER2–ABC. The PALOMA-4 study evaluated the effectiveness and safety of PAL + LET in Asian patients.
Methods
PALOMA-4 is an international, double-blind, phase 3 trial that randomizes postmenopausal Asian women who have not previously received ER+/HER2– ABC systemic therapy to receive PAL at a 1:1 ratio (oral 125 mg/d; 3 weeks After that, rest for 1 week) + LET (2.5 mg/d orally; continuous administration) or PBO + LET.
The primary endpoint was a Kaplan-Meier analysis of the investigator-assessed progression-free survival (PFS); comparisons between arms used the stratified log-rank test. Secondary endpoints include objective response rate (ORR) and safety; the Cochran-Mantel-Haenszel test was used for comparison between arms. Descriptively summarizes security.
Results
The enrolled patients (N = 340) were randomly assigned to each group (PAL + LET, 169; PBO + LET, 171). The median follow-up time for overall survival was 52.8 months. The baseline characteristics between the two groups were generally similar. At the time of the data cutoff (August 31, 2020), the median PFS of PAL + LET was 21.5 months, and PBO + LET was 13.9 months (HR=0.68, 95% CI: 0.53–0.87; P = 0.0012). T
he ORR based on the investigator’s assessment was 37.3% and 31.6% in all patients (P = 0.154), and 43.4% and 38.0% in patients with measurable diseases (P = 0.206).
The most common grade 3/4 adverse events (AE) of PAL + LET and PBO + LET were neutropenia (84.5% vs 1.2%), leukopenia (36.3% vs 0.6%), and thrombocytopenia (6.5 % vs 0.6%) and anemia (4.8% vs 1.8%). Febrile neutropenia was reported using only PAL + LET (2.4%). The discontinuation rate due to AEs was 7%.
Conclusion
PALOMA-4 is the largest CDK 4/6 inhibitor study conducted in Asian ABC patients so far, and it has confirmed the effectiveness and safety of PAL + LET as the first-line treatment of ER+/HER2–ABC in postmenopausal Asian women.
Clinical trial identification: NCT02297438.
Xu BH, et al. PALOMA-4: Primary results from a phase III trial of palbociclib (PAL) + letrozole (LET) vs placebo (PBO) + LET in Asian postmenopausal women with estrogen receptor–positive/human epidermal growth factor receptor 2 –Negative (ER+/HER2–) advanced breast cancer (ABC). Annals of Oncology (2021) 32 (suppl_5): S457-S515. 10.1016/annonc/annonc689.
The final result of the PEARL study: Pabociclib + endocrine therapy (ET) and capecitabine (CAP) in HR+/HER2- metastatic breast cancer (MBC) progressing with aromatase inhibitors (AIs) Overall survival (OS)
Background
The PEARL study did not show the advantages of P+ET and (vs) CAP in the progression-free survival (PFS) of AI-resistant MBC patients, but P+ET was better tolerated and showed a significant delay in quality of life deterioration. The final research result data is reported here.
Methods
PEARL has two consecutive cohorts: Cohort 1 (C1) has 296 patients randomly assigned to the P+ exemestane and CAP groups, and cohort 2 (C2) has 305 patients randomly assigned to P+ Fulvestrant (F) and CAP group. Secondary endpoints include OS in C2 and wild-type (wt) ESR1 (measured in baseline ctDNA) (C1+C2) patients.
It is planned to perform OS analysis when 152 deaths in C2 occur, so as to have 80% power to detect the increase in OS from 22 months (m) to 33 months (m) in wtESR1 patients (P+F or P+ET) . The adjusted hazard ratio (aHR) was calculated using a stratified Cox proportional hazard model, with the treatment group, stratification factors, and number of affected sites as covariates.
Results
At the time of the data cutoff (January 11, 2021), the median follow-up time for C2 and wtESR1 patients were 28.0 m and 30.3 m, respectively. The median OS of C2 was 31.1 m (P+F), while the CAP was 32.8 m (aHR=1.10, 95% CI: 0.81–1.50; p = 0.550). The median OS of patients with wtESR1 was 37.2 m for P+ET and 34.8 m for CAP (aHR=1.06, 95% CI: 0.81-1.37; p = 0.683).
Subgroup analysis did not show the advantages of P+ET and CAP in OS. 79.8% and 82.9% of P+ET and CAP patients received follow-up treatment, respectively.
The median of subsequent lines in the P+ET group is 3 (1-10), and in the CAP group it is 3 (1-9). 26.1% of patients in the CAP group received CDK4/6 inhibitor + ET, and 36.1% of patients in the P+ET group received CAP. The median PFS2 was defined as the time from randomization to the end of the first follow-up treatment or death.
The C2 of the two groups was similar, with a P+F of 18.3 m and a CAP of 17.7 m (aHR=0.95, 95% CI: 0.73-1.25; p = 0.728). In patients with wtESR1, P+ET was 18.3 m and CAP was 18.2 m (aHR=1.04, 95% CI: 0.83-1.31; P= 0.717). In this final analysis, there is no change in PFS and response. No new safety findings were observed during the longer follow-up.
Conclusion
Among MBC patients with AI progression, Palbociclib + endocrine therapy did not show a statistically superior OS compared to CAP.
Clinical trial identification: NCT02028507.
Jimenez MM, et al. Overall survival (OS) of palbociclib (P) plus endocrine therapy (ET) versus capecitabine (CAP) in hormone-receptor+/HER2- metastatic breast cancer (MBC) that progressed on aromatase inhibitors (AIs): Final results of the PEARL study. Annals of Oncology (2021) 32 (suppl_5): S457-S515. 10.1016/annonc/annonc689.
Palbociclib dose pattern in Swedish patients with metastatic breast cancer: evidence from the SIRI study
Background
Palbociclib is a cyclin-dependent kinase (CDK) 4/6 inhibitor, suitable for use with aromatase inhibitors or Fulvestrant in HR+/HER2-metastatic breast cancer (MBC) patients). The Swedish Ibrance Registries Insights (SIRI) study used a Swedish national cohort of MBC patients treated with palbociclib to investigate real-world dose patterns.
Methods
This is a retrospective study using a population-based Swedish health data registry. This cohort includes all patients ≥ 18 years of age and ≥ 1 prescription of palbociclib between January 2017 and June 2020. The minimum follow-up time is 3 months. Studied the whole group in general and over time, the starting dose and dose changes in the subgroups.
Results
1,226 patients who were prescribed palbociclib were identified, of which 10 were men. The mean (SD) age at the start of treatment was 65 (11) years. 11% of patients have new-onset MBC.
Most patients start taking 125 mg (86.8%), the proportion of elderly patients is low (80%), and the proportion declines over time (table). 43.5% of patients had a dose reduction ≥ 1 time, and the share decreased over time (47.1% in 2017; 35.2% in 2020).
The proportion of patients who started to reduce from 125 mg to 100 mg and 75 mg (final dose) was 26.6% and 19.4%, respectively, and the proportion of patients who started from 100 mg to 75 mg was 28%. The main endocrine therapy does not affect the dosage pattern.
Younger patients (<50 years old) start with 125 mg, and more frequently reduce the dose to the final dose of 100 mg (34% vs 25.7% for 50-69 years old, 26.1% for age ≥ 70 years old), and the dose is from 125 mg Reducing to 75 mg will increase with age (<50: 12%; 50-69: 18%; ≥ 70: 23.6%).
Conclusion
Most patients treated with palbociclib in Sweden started with the recommended starting dose, but a trend of decreasing starting dose was observed over time. In general, dose reduction seems to be more common in clinical practice, but as time goes by, the trend toward clinical trial results has shown a downward trend.
Valachis A, et al. Palbociclib dose patterns in Swedish patients with metastatic breast cancer: Evidence from the SIRI study. Annals of Oncology (2021) 32 (suppl_5): S457-S515. 10.1016/annonc/annonc689
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



