Some FDA-approved drugs are expected to be re-used to treat COVID-19
- Did Cloud Seeding Unleash a Deluge in Dubai?
- News draftScientists Identify Gut Bacteria and Metabolites that Lower Diabetes RiskNews draft
- OpenAI’s Model Matches Doctors in Assessing Eye Conditions
- UK: A Smoke-Free Generation by Banning Sales to Those Born After 2009
- Deadly Mutation: A New Monkeypox Variant Emerges in the DRC
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
Some FDA-approved drugs are expected to be re-used to treat COVID-19
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
PLoS Pathog: Some FDA-approved drugs are expected to be re-used to treat COVID-19.
In a new study, Adam Pickard and Karl Kadler of the University of Manchester in the United Kingdom and their colleagues discovered that some drugs approved by the U.S. Food and Drug Administration may be safely reused to treat COVID-19 infections.
Although the SARS-CoV-2 vaccine has been developed, effective therapeutic drugs are needed before global immunization can be achieved. In a new study, Adam Pickard and Karl Kadler of the University of Manchester in the United Kingdom and their colleagues discovered that some drugs approved by the U.S. Food and Drug Administration ( FDA ) may be safely reused to treat COVID-19 infections.
The relevant research results were published in the journal PLoS Pathogens on September 9, 2021. The title of the paper is ” Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells .”

Most of the world’s population is still not vaccinated, but few drugs have proven to be safe, easy to dispense, and can reduce the spread of SARS-CoV-2.
In order to determine drugs that can effectively treat SARS-CoV-2 infection, these authors screened 1971 FDA- approved therapeutic drugs using a luminescent enzyme-labeled version of SARS-CoV-2 virus to quantitatively determine viral load.
They then analyzed the efficacy of these drugs in a range of infected human cell types and observed how the virus replicated in infected cells after exposure to each drug.
These authors determined that nine drugs can effectively inhibit virus replication in cells that have been infected with SARS-CoV-2.
They are atovaquone, Manidipine, and Manidipine 2HCl. , Amodiaquine dihydrochloride dihydrate (amodiaquine dihydrochloride dihydrate), abemaciclib (also known as LY2835219), panobinostat (also known as LBH589), bedaquiline (bedaquiline), Vitamin D3 and ebastine.
However, this study has its limitations because it was only conducted in human cells, and the efficacy of these drugs in treating patients with SARS-CoV-2 infection has yet to be tested.
The need for clinical trials to determine whether these drugs are suitable for the treatment of patients with COVID-19.
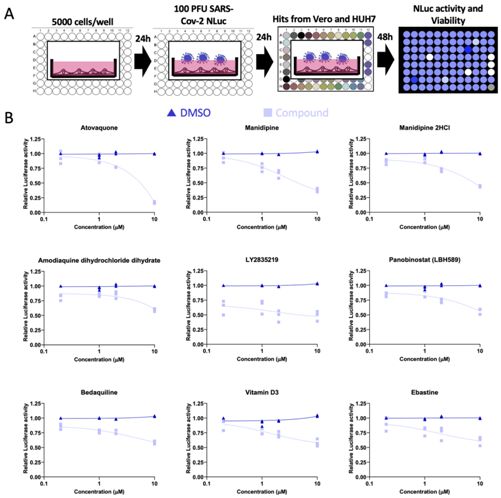
Picture from PLoS Pathogens, 2021, doi:10.1371/journal.ppat.1009840.
These authors said, “Our research has identified compounds that are safe for humans and has shown effectiveness in reducing SARS-CoV-2 infection and replication in human cells.
Because these drugs are FDA approved and have established The safe doses used on patients, so clinical trials of these drugs can be initiated in a relatively short period of time .”
Kadler added, “We have identified drugs that can prevent the SARS-CoV-2 virus (which is the coronavirus of COVID-19) from replicating in human cells cultured in vitro.
These drugs include FDA approval for the treatment of Pneumocystis jiroveci (Pneumocystis carinii) ebastine for pneumonia, and vitamin D3 available over the counter, and they may be a powerful supplement for the treatment of COVID-19.
However, these drugs have not been evaluated in COVID-19 patients, and they are not current drugs. Or an alternative to the vaccination plan.”
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



