15-valent pneumonia vaccine: Merck Vaxneuvance approved by EU soon
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
15-valent pneumonia vaccine: Merck Vaxneuvance approved by EU soon
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
15-valent pneumonia vaccine: Merck Vaxneuvance approved by EU soon.
15-valent pneumonia vaccine! Merck’s Vaxneuvance (V114) is about to be approved in the EU: it is used in adults ≥18 years of age to prevent invasive pneumococcal disease!
Vaxneuvance was approved by the US FDA in July this year. The vaccine is composed of pneumococcal polysaccharides from 15 serotypes combined with CRM197 carrier protein, including 22F and 33F serotypes.
Merck recently announced that the European Medicines Agency (EMA) Committee for Human Medicinal Products (CHMP) has issued a positive review opinion recommending the approval of Vaxneuvance (15 Valence pneumococcal conjugate vaccine, V114): It is used in adults aged 18 years and above for active immunization to prevent 15 kinds of Streptococcus pneumoniae serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14 , 18C, 19A, 19F, 22F, 23F, 33F) caused by invasive pneumococcal disease (IPD).
Now, CHMP’s opinions will be submitted to the European Commission (EC) for review, which is expected to make a final review decision by the end of this year.
In the United States, Vaxneuvance was approved in July this year for active immunization in adult populations of 18 years and older to prevent invasive diseases caused by the 15 serotypes of Streptococcus pneumoniae covered by the vaccine.
Pneumococcal disease is an infection caused by Streptococcus pneumoniae. The different strains of this bacteria are called serotypes. Invasive pneumococcal disease (IPD) occurs when Streptococcus pneumoniae invades parts of the body where bacteria are not normally present . Approximately 80% of the adult IPD burden occurs in people 50 years and older.
Globally, the incidence of pneumococcal disease in adults is on the rise, in part driven by pathogenic serotypes that are not covered by currently available pneumococcal conjugate vaccines. Serotypes 3, 22F and 33F have a significant impact on the burden of IPD.
Vaxneuvance is a 15-valent vaccine consisting of pneumococcal polysaccharides from 15 serotypes combined with CRM197 carrier protein, including 22F and 33F serotypes. These two serotypes are usually associated with invasive pneumococcal diseases worldwide . But it is not included in the pneumococcal conjugate vaccine currently approved for use in adults.
Currently, Vaxneuvance is also in phase 3 clinical development for the prevention of pneumococcal disease in children. Previously, the US FDA has granted Vaxneuvance Breakthrough Drug Designation (BTD) for use in pediatric populations from 6 weeks to 18 years old and adults aged 18 years and older to prevent invasive pneumococcal disease (IPD) caused by vaccine serotypes.

Pneumococcal pneumonia (Image source: bigstockphoto.com)
The FDA’s approval of Vaxneuvance and the positive review of CHMP are based on data from 7 randomized, double-blind clinical studies. These studies evaluated the safety, tolerability and immunogenicity of Vaxneuvance in the adult population.
Clinical data shows that for the 13 common serotypes, Vaxneuvance induces an immune response not lower than the currently available 13-valent pneumococcal conjugate vaccine (PCV13, namely: Prevnar 13 [Prevnar 13]), which is achieved through photophagocytic activity (OPA). ) The geometric mean titer (GMT) is evaluated.
In addition, for the common serotype 3 and the two serotypes 22F and 33F unique to Vaxneuvance, the immune response induced by Vaxneuvance is better than that of PCV13 .
In the key phase 3 PNEU-AGE (V114-019) study, the superiority of Vaxneuvance over PCV13 is based on the comparison of serotype 22F (GMT ratio=32.52[95%CI:25.87, 40.88]) and serotype 33F (GMT ratio) =7.19[95%CI:6.13,8.43]) OPA-GMT ratio is statistically significantly higher, and a key secondary target for serotype 3 (GMT ratio=1.62[95%CI: 1.40, 1.87]) evaluation of. Randomized controlled trials have not been conducted to evaluate the clinical effectiveness of Vaxneuvance and PCV13.
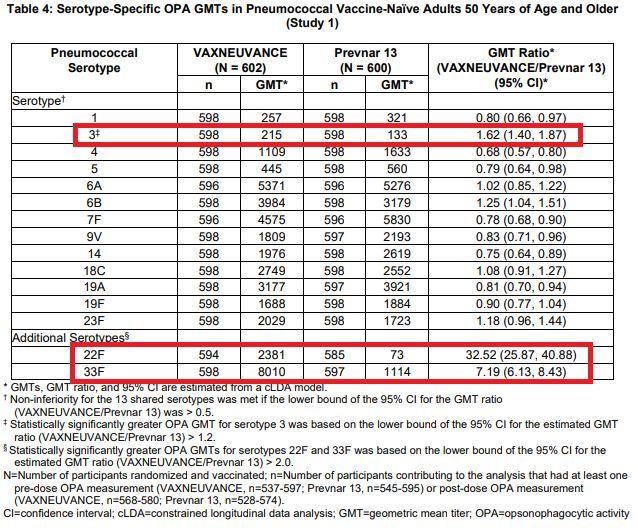
PNEU-AGE (V114-019) research data
There are more than 90 different types of pneumococcal bacteria, and their effects on adults are different from children. Pneumococcal serotypes (such as 22F and 33F) that are not included in the currently marketed conjugate vaccines are usually associated with invasive pneumococcal diseases worldwide.
At present, 13% of invasive pneumococcal diseases in adults aged 65 and over in the United States are caused by serotypes 22F and 33F. In Europe, adult pneumococcal diseases caused by these two serotypes account for 7%-12%.
In addition, serotype 3 remains one of the main causes of invasive pneumococcal disease in adults and children, although it has been included in currently available pneumococcal vaccines.
In the United States, 15% of invasive pneumococcal disease among adults aged 65 years and older is still caused by serotype 3; this proportion accounts for 12%-18% of adults in European countries.
Vaxneuvance’s Phase 3 clinical development project consists of 16 clinical trials , which studied the safety of Vaxneuvance in different groups of people (including healthy elderly people, healthy children, people with impaired immune function, and people with certain chronic diseases). Tolerability and immunogenicity.
15-valent pneumonia vaccine: Merck Vaxneuvance approved by EU soon
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



