Are 28 million children in US really benefit from FDA approval of vaccines?
- Engineered Soybeans with Pig Protein: A Promising Alternative or Pandora’s Dish?
- Severe Fever with Thrombocytopenia Syndrome (SFTS): A Tick-Borne Threat with High Mortality
- Why Isolating Bananas Extends Their Shelf Life?
- This common vitamin benefits the brain and prevents cognitive decline
- New report reveals Nestlé adding sugar to infant formula sold in poor countries
- Did Cloud Seeding Unleash a Deluge in Dubai?
Are 28 million children in US really benefit from FDA approval of vaccines?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Nature Hot Discussion: Are 28 million children in US really benefit from FDA approval of vaccines? What is the role and significance of children’s vaccinations against the COVID-19?
On October 29, the U.S. Food and Drug Administration approved the emergency use authorization of the COVID-19 vaccine jointly developed by Pfizer Pharmaceuticals and the German Biotech Company for children aged 5 to 11 years old in the United States, which will enable 28 million children in the United States to use it. Start to get the COVID-19 vaccine.
According to Pfizer, its COVID-19 vaccine for children is one-third of the adult vaccine dose.
The Centers for Disease Control and Prevention is expected to convene an advisory committee meeting next week to review the dosage of pediatric medications, and then sign approval for the rapid distribution of the vaccine to the public.
Recently, scientists have explored what effect can children vaccinate against the COVID-19 epidemic bring about? Does it make sense? This article will take you to think about this question…
When it comes to vaccinating children and adolescents with the COVID-19, the three questions most parents will raise are:
“Is this vaccine effective for children? Is it safe?”
“What is the adverse reaction of the child?”
“When will my city open?”
There are many reasons for the late arrival of the COVID-19 vaccine for minors. One of them is that children are not the most vulnerable group.
Although compared with adults, there are still a small number of minors who are at risk of developing severe illness after being infected with the new coronavirus, but most of the clinical manifestations are usually mild or asymptomatic.
For example, a recent observational study published in Southeast Asia and South Asia and hospital reports in 7 countries showed that 40% of children diagnosed as asymptomatic are asymptomatic.
A study of seven countries published in The Lancet estimated that during the pandemic, less than 2 children out of 1 million children died of the COVID-19.
Therefore, the elderly, as a higher-risk group, have priority in obtaining the right to vaccinate.
In addition, the risk and uncertainty of children’s vaccination are higher, which is one of the reasons for the postponement of open vaccination.
Despite this, children are still at risk of contracting the COVID-19 virus, and may still get sick from being infected with the COVID-19; as an asymptomatic infected person after infection, it is easier to silently spread the COVID-19 to others.
What is more noteworthy is that infectious disease scientist Dr. Anthony Fauci also mentioned that children may be more susceptible to Delta mutant strains than Alpha mutant strains; as we all know, Delta mutant strains are more infectious.
Therefore, after the adult COVID-19 vaccination rate continues to rise and the virus continues to mutate, it is imperative for minors to be vaccinated.
At the Mexico City library, a minor is receiving the first dose of Pfizer’s COVID-19 vaccine. According to the prediction of the modelers, vaccinating children with the COVID-19 vaccine can significantly inhibit the spread of any new coronavirus variants
In the United States, with the approval of the US Food and Drug Administration to vaccinate children between 5 and 11 years old, scientists have conducted in-depth discussions on this issue. They hope to understand and study the effect of vaccinating children on the COVID-19 pandemic. .
A few days before the U.S. Food and Drug Administration made this decision, an agency’s advisory board reviewed data from a clinical trial of a low-dose Pfizer biotechnology vaccine for children of this age, and almost all voted to recommend that the FDA urgently approve.
Relevant personnel predict that the US Centers for Disease Control and Prevention will also approve of this measure. The approval and approval of the US Centers for Disease Control and Prevention will be the last necessary step for the COVID-19 vaccine to be vaccinated among children. In a few weeks, vaccination will begin among children. Infectious disease researchers are thinking and looking forward to the question: How to vaccinate these 5-11-year-old groups with the COVID-19 vaccine and let them gain immunity to the virus will bring positive effects and significance to the COVID-19 epidemic that is ravaging mankind ? This group is currently the largest group of people in the United States that has not yet been eligible for vaccination.
Emma McBryde is an infectious disease modeler at the Australian Institute of Tropical Health and Medical Research. She thinks so about this issue: “This action will save the lives of this group of people. Of course, it may also have a wider impact. Because in the past few months, many American children between the ages of 5 and 11 have returned to school without being vaccinated. The results of this are tragic. This group now accounts for a large part of the COVID-19 pneumonia cases, and There is also the ability to spread the virus to others. Therefore, if this group is not vaccinated, the outcome is simply a vicious circle. Therefore, we believe that while saving the life of a child, it must mean saving the lives of more adults. .”
Child vaccination-the advantages outweigh the disadvantages
The FDA advisory team voted to approve the vaccine on October 26, based on clinical trial data showing that Pfizer Biotech’s vaccine is approximately 91% effective in preventing COVID-19 infections in children aged 5 to 11. Approximately 4,650 children participated in the trial; nearly two-thirds of these children received one-third the dose of the vaccine as an adult (other people were given a placebo).
This trial is very similar to the US trial of vaccinating adults with mRNA vaccines. In this trial, these children received two doses of the vaccine three weeks apart.
For those children who have undergone trials, the final data show that such a COVID-19 vaccine is safe. MRNA-based vaccines lead to a small risk of myocarditis (an inflammation of the heart muscle) and pericarditis (an inflammation of the inner lining around the heart), especially in young men.
However, Andrew Pavia, director of the Department of Pediatric Infectious Diseases at the University of Utah Health Center in Salt Lake City, said: “There are no reports of these two conditions among the children aged 5 to 11 who participated in the trial. This is a very good sign. However, Pavia pointed out that, If this vaccine is distributed to more people, regulators will need to observe for any signs of side effects.”
Relevant personnel predict that the US Centers for Disease Control and Prevention will also approve of this measure. The approval and approval of the US Centers for Disease Control and Prevention will be the last necessary step for the COVID-19 vaccine to be vaccinated among children. In a few weeks, vaccination will begin among children.
Infectious disease researchers are thinking and looking forward to the question: How to vaccinate these 5-11-year-old groups with the COVID-19 vaccine and let them gain immunity to the virus will bring positive effects and significance to the COVID-19 epidemic that is ravaging mankind ? This group is currently the largest group of people in the United States that has not yet been eligible for vaccination.
Before the consultant team started this meeting, the U.S. Food and Drug Administration conducted an independent review of Pfizer’s data and evaluated 6 fictitious U.S. scenarios in which there are varying degrees of virus in the community, and they found that: in most cases Among them, the benefits of central tube vaccines are obviously greater than the negative risks it brings.
Even if the speed and level of the spread of the new coronavirus in the United States is already very low, the benefits of the new coronavirus vaccine may still outweigh the potential risk of causing heart problems, because these heart problems are usually within a few days or a short time after vaccination. It will get better internally, but the new coronavirus is a deadly virus.
Although the COVID-19 virus is not as deadly among young people as it is among the elderly, according to the US Centers for Disease Control and Prevention data: about 440 children aged 5 to 18 in the United States have died from the COVID-19 virus; and the number of people infected with the COVID-19 in all age groups About 724,000. For people in the 5-18 age group, students return to school and then contract the Delta mutant virus, which has led to a sharp increase in pediatric cases starting in late July.
According to a report from the American Academy of Pediatrics, since the beginning of the epidemic, 6.3 million American children have tested positive for the COVID-19, and nearly one-third of them were diagnosed within the 11 weeks ending October 21.
Pavia said: “For me, the harm of the Delta mutant virus to children of this age will not exceed the side effects that the COVID-19 vaccine may bring to them. I think it is not difficult for this group to get the COVID-19 vaccination approval.”
Look to the future
Since the improvement of the Delta mutant virus epidemic in September, the number of COVID-19 infections in the United States has been declining. Most modelers predict that regardless of whether the Pfizer vaccine is finally authorized for vaccination of children aged 5 to 11, this trend of declining number of people infected with the COVID-19 will continue until early 2022.
Katriona Shea, an applied theory ecologist at Penn State University Park, said: “In other words, the world is attacked by another new variant of the new coronavirus, it is like giving us a slap in the face of this modeling system.”
Child effect: Simulate the effect of vaccinating the COVID-19 vaccine for children aged 5 to 11 years in the United States in early November 2021
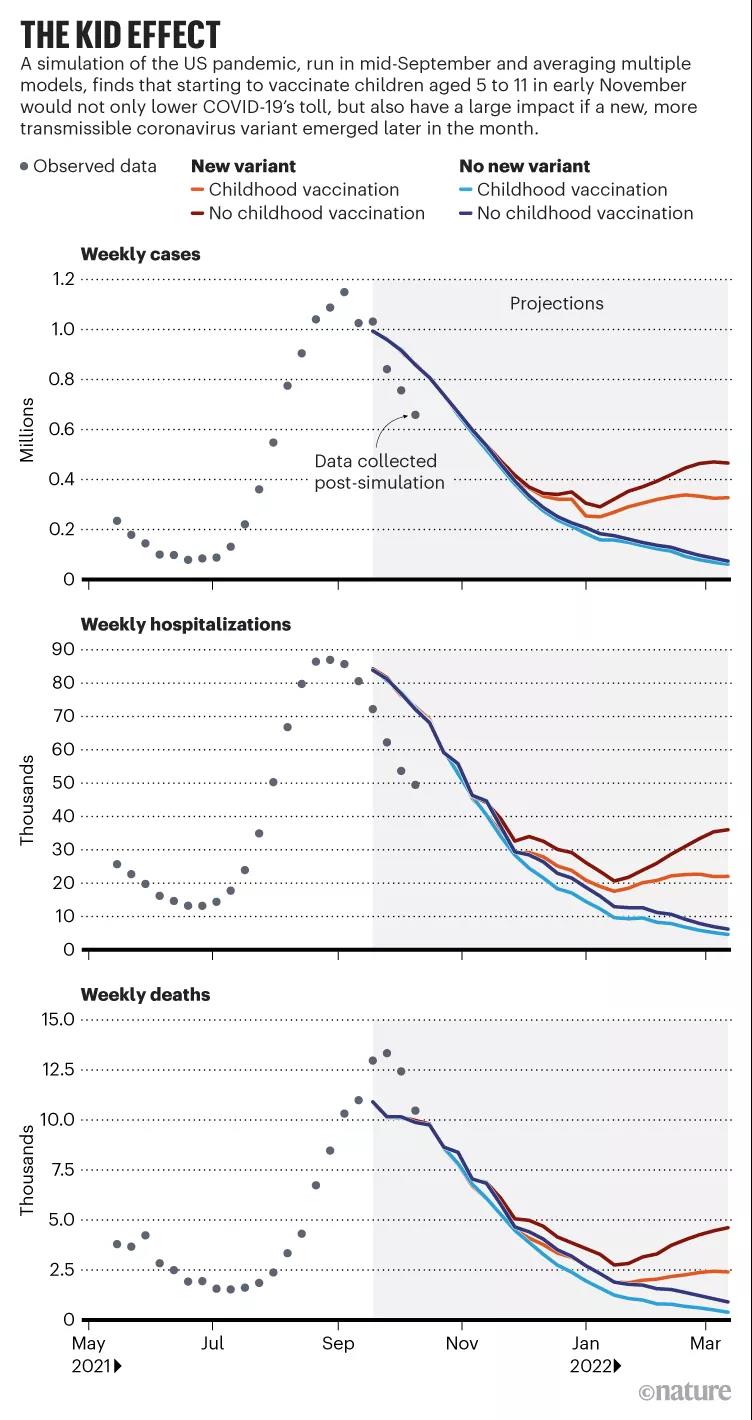
Shea is the co-director of the COVID-19 Scenario Modeling Center, which released its ninth pandemic trajectory prediction in September, which considered how vaccines for children aged 5-11 might affect new infections and deaths in the United States . Shea said: “This prediction is based on averaging the predictions of nine other modeling teams.
It shows that although vaccination of children will bring a lower number of cases, as long as we are lucky enough, we will not encounter There will be no huge differences in population levels for other mutant viruses after Delta.”
However, there are also data showing that if a new mutant virus arrives in mid-November, children’s vaccination may affect the course of the new coronavirus pandemic in the United States. huge influence.
In preparation for the possible vaccination approved by US regulators, the White House announced a plan last week to distribute low-dose vaccines to pediatrician offices, hospitals and pharmacies.
However, even if Pfizer Biotech’s vaccine is fully approved for emergency use, it remains to be seen how children in the 5-11 age group feel about vaccination and whether their parents will allow them to be vaccinated. Mina Fazel is a child and adolescent psychiatrist at the University of Oxford in the United Kingdom.
She and her colleagues surveyed nearly 28,000 students between the ages of 9 and 18 in 180 schools in the UK and found that younger children are better than older children. He is more hesitant about vaccination.
The survey also shows that social media also has a certain amount of credit for this: students who spend more than 4 hours a day on social platforms are less willing to be vaccinated than those who spend less time on social media. Fazel said: “This generation of young people is being exposed to new information on an unprecedented scale. It seems that tailoring public health activities for children and providing a platform for the input of their information is more important than ever. .”
The global impact of child vaccination
It remains to be seen what the vaccination authorization for children aged 5-11 in the United States means on a global scale. Now, nearly 70 countries have vaccinated less than one-fifth of the population with the COVID-19 vaccine, and it may not be vaccinated for children younger than that in the next few months or even years; but including Israel Some countries are waiting for the decision of US regulators before approving their own vaccinations.
However, other countries are already vaccinating children under 12 years of age. For example, Chile, China, Cuba, and the United Arab Emirates have all started vaccinating children with various vaccines that can deal with the new coronavirus in the past three months.
According to McBryde, in some areas, people’s natural immunity to the COVID-19 virus is relatively low and they are vulnerable to the COVID-19 virus, because the spread of the virus in these communities has been very low during the entire world-wide pandemic of the COVID-19 epidemic. Therefore, child vaccination is very important for these regions and countries. For example, Australia plans to reopen its international borders in November, allowing citizens and permanent residents to enter or exit the country when the vaccination rate in their state of residence reaches 80%. This move will “attract the virus” to enter the United States, so it is necessary to increase people’s immunity to the virus through vaccination and “soften the landing” as much as possible. This also includes vaccinating children. However, vaccination for children under 12 has not yet been submitted to Australian regulators for approval.
On October 25th, Moderna, a vaccine manufacturer based in Cambridge, Massachusetts, stated: “The low-dose mRNA vaccine for children aged 6 to 11 is safe and effective!” The company has not yet applied for approval from the U.S. Food and Drug Administration. . According to a statement issued by Pfizer’s chief executive officer at an event held by The Atlantic last month, the company’s vaccination data for children under 5 years of age is expected to be released before the end of this year. Moderna is also experimenting with COVID-19 vaccination in 6-month-old children.
The researchers recorded the above-mentioned different experts’ thinking on this issue in an article titled “What COVID vaccines for young kids could mean for the pandemic” in “Nature” magazine:

Reference:
https://www.nature.com/articles/d41586-021-02947-z
Are 28 million children in US about to benefit from FDA approval of vaccines?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



