JACC: What are the hidden worries about CAR-T cell therapy?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
JACC: What are the hidden worries about CAR-T cell therapy?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
JACC: What are the hidden worries about CAR-T cell therapy?
The hidden worries under the prestige! What has been discovered in the biggest research after the launch of CAR-T cell therapy?
Chimeric antigen receptor T (CAR-T) cells are one of the most rapidly developing tumor immunotherapy methods in recent years.
By “engineering” T cells in vitro, T cells can specifically recognize tumor antigens and kill tumor cells in patients. he efficacy of CAR-T cells has been confirmed in a variety of hematological tumors[1-3] The survival benefits brought to patients with refractory acute B lymphocytic leukemia and other tumors are surprising, and the clinical application of CAR-T therapy is also increasing year by year [4].
But there is no lack of shadows behind the halo. CAR-T cells can cause a series of complications such as cytokine release syndrome (CRS) and immune effector cell-related neurotoxic syndrome.
Early data confirmed that more than 10% of patients receiving CAR-T cell therapy had cardiovascular and pulmonary adverse events (CPAEs) [5-6].
However, the subjects of clinical trials may have a certain selection bias, and the complications of the cardiopulmonary organs caused by CAR-T cells still lack strong research evidence .
Recently, Professor Goldman of Leviev Heart Center, Chaim Sheba Medical Center, Israel, and his research team, published a research result of important clinical significance in the Journal of the American College of Cardiology (JACC) [7].
The Goldman team conducted a retrospective analysis of 2657 patients who received CAR-T cell therapy from July 2014 to June 2020 and found that: CAR-T cell therapy is associated with hypotension, cardiomyopathy, tachyarrhythmia, The reports of various CPAEs such as pericardial disease, cardiogenic shock, respiratory failure and pleural disease are increasing .
Some patients with CPAEs also reported CRS, a common adverse reaction of CAR-T cells. Among patients with high risk factors for cardiovascular disease, the report rate of CAR-T cell-related CPAEs is higher, and the mortality of patients after CPAEs is higher than that of patients after CRS .
This research result is also a reminder to clinicians and researchers that while paying attention to the common adverse reactions of CAR-T cells, the cardiopulmonary toxicity should not be ignored .
In the process of patient treatment, clinicians should adopt close monitoring and active treatment methods to identify and treat patients’ CAR-T cell-related CPAEs early in order to improve the patient’s clinical outcome.

Next, let’s take a look at how Professor Goldman’s team carried out this research.
Professor Goldman’s team used the FDA’s adverse event reporting system database to design an observational and retrospective pharmacovigilance study, which analyzed the period from July 2014 to June 2020 when receiving any of Axicabtagene and Tisagenlecleucel targeting CD19. CAR-T cell product treatment, adverse events (AEs) in patients ≥16 years of age .
Researchers used the latest medical records after deduplication to code the AEs using the medical dictionary of supervision activities, and then grouped them by disease.
Tachyarrhythmias, cardiomyopathy, and cardiogenic shock are defined as severe AEs.
The researchers also used data registered by the International Blood and Bone Marrow Transplant Research Center to estimate the total number of people receiving CAR-T cell therapy in the United States.
The researchers also used the reported odds ratio method (ROR) and the information component method (IC) in the ratio imbalance analysis method to assess the association between over-reported AEs and CAR-T cells and different AEs; logistic regression models were used for different ages Confounding factors such as, gender, and CAR-T cell type were corrected.
A total of 2657 patients were enrolled in this study, of which 65.2% were treated with Axicabtagene.
Overall, 54.8% and 20.5% of patients had CRS and CPAEs, respectively, and 68.3% of patients who had CPAEs had CRS at the same time .
The proportion of different types of CPAEs combined with CRS fluctuates between 50.7%-100%, and all CPAEs are positively associated with CRS .
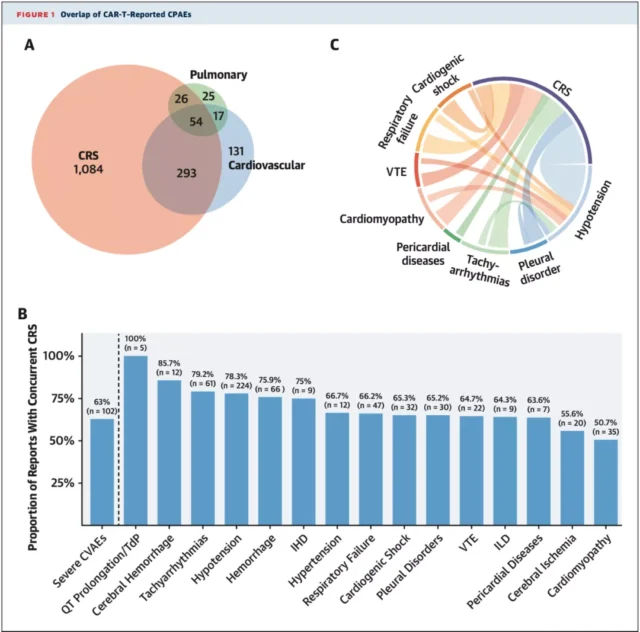
AEs after CAR-T cell therapy and their correlation
In addition, CAR-T cell therapy is associated with increased reports of CPAEs such as hypotension, cardiomyopathy, tachyarrhythmia, pericardial disease, cardiogenic shock, respiratory failure and pleural disease.
Cardiorenal syndrome is the most common in cardiomyopathy reports; atrial fibrillation and ventricular arrhythmia are the most important tachyarrhythmias.
Compared with Tisagenlecleucel, the report rate of CRS, tachyarrhythmia and venous thromboembolism (VTE) is higher in patients treated with Axicabtagene, and ventricular arrhythmia and venous thromboembolism are only related to Axicabtagene treatment, but serious AEs are in two The reporting rates are similar among the products.
The Goldman team also found that the mortality rate of patients after CPAEs was higher than that of patients with CRS (30.9% vs 17.4%), and the mortality rate of central shock was as high as 80.9% .
Among people with high risk factors for cardiovascular disease, the report rate of CAR-T cell-related CPAEs is higher.
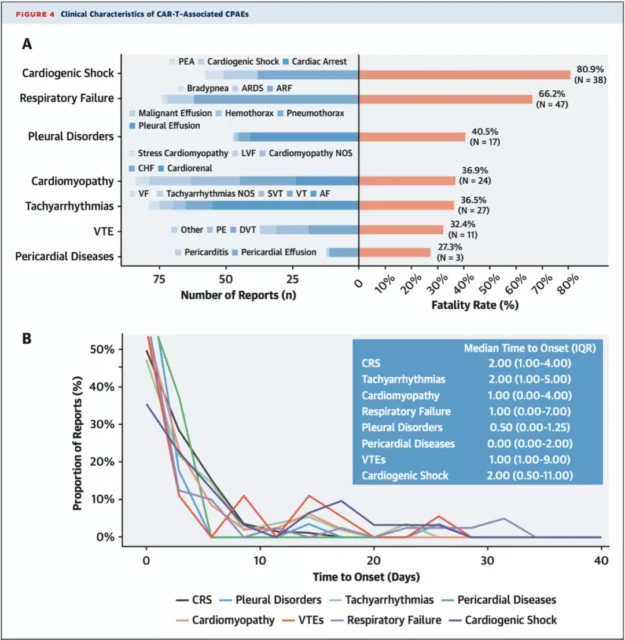
Common CPAEs and corresponding lethality (A), the occurrence time of common CAR-T cell-related AEs (B)
It should be pointed out that most CPAEs occur in the first 10 days after CAR-T cell infusion, but some CPAEs occur later, such as VTE and cardiogenic shock.
It is worth mentioning that the source of data for this study higher quality FDA Adverse Event Reporting System database, is the commercial CD19-targeted therapies listed CAR-T cells, the most comprehensive description of the characteristics of CPAEs , to provide a more High-quality research evidence and credible conclusions confirm the association between CAR-T cell therapy and a variety of CPAEs.
This study also compared the reporting rates of CRS and CPAEs between different products and the lethality rates of different CPAEs, providing valuable reference data for the subsequent clinical application of CAR-T cell therapy.
The extensive intersection of CRS and CPAEs may be the result of cytokine toxicity during CAR-T cell therapy.
In view of the cardiopulmonary toxicity of CAR-T cell therapy may bring adverse effects to many patients, clinicians and scientific researchers need to pay attention to this phenomenon.
In addition, this study described in detail the severity, time and characteristics of CAR-T cell-related cardiotoxicity.
Before receiving CAR-T cells, clinicians should comprehensively evaluate the patient’s organ function.
For people with underlying diseases or high-risk factors for cardiovascular disease, they should give corresponding treatment to enhance their cardiopulmonary function.
After the start of treatment, prospective drug application and close monitoring may be able to prevent the occurrence of CPAEs, thereby improving the clinical outcome of patients.
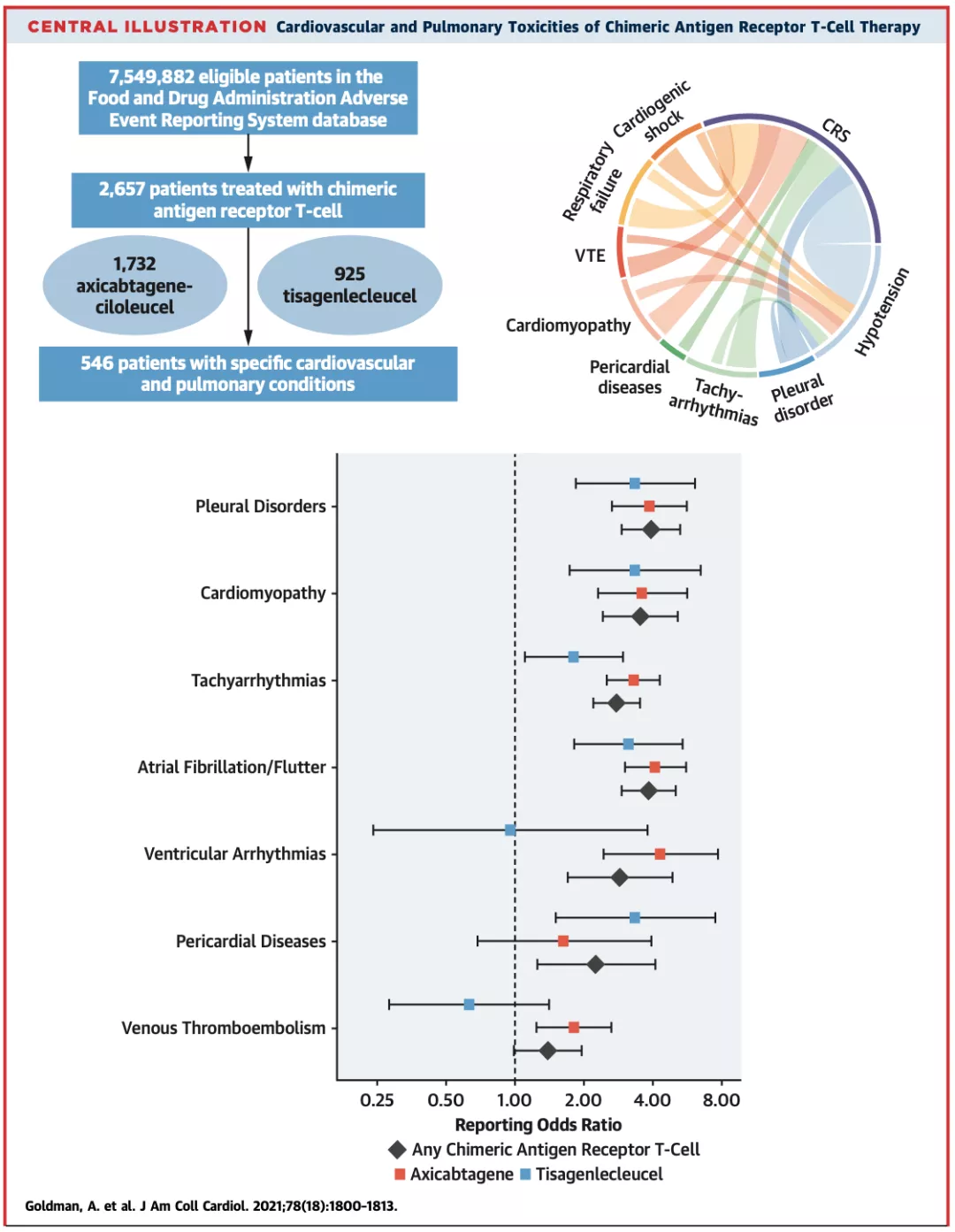
Of course, this study also has certain limitations. For example, AEs in the database of the adverse event reporting system rely on voluntary reporting, and the proportion of reports may be low.
In addition, the total number of people receiving CAR-T cell therapy is unknown. Therefore, this study failed to calculate the actual incidence of CPAEs, and the comparison between different CAR-T cell products is only at the theoretical level.
In general, while using CAR-T cells that are full of endless possibilities, researchers should also summarize and reflect in time. The new therapy may be a double-edged sword before the mechanism is fully clarified.
Meticulous clinical research and post-marketing efficacy/complication monitoring feedback will help to clarify the possible “off-target effects” of these star therapies, and then build on the original basis Continuously optimize and adjust .
The exploration of CPAEs mechanism and the screening of high-risk groups of CAR-T cell therapy complications are also scientific issues worthy of research in the future.
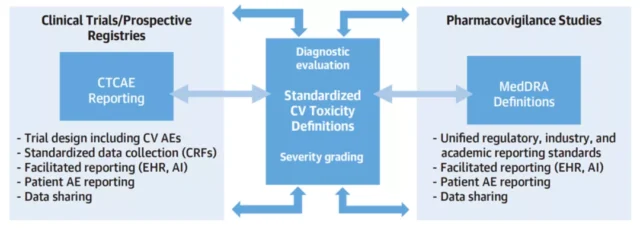
Improved AE reporting mechanism [8] requires the participation of the industry
We believe that with the joint efforts of researchers, clinicians, and patients, the application and promotion of CAR-T, and the evaluation of benefits and risks will be more scientific and reasonable.
Also hope that this powerful weapon born in genetic engineering can help more The patient was reborn in despair.
References
[1] Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531-2544. doi:10.1056/NEJMoa1707447
[2] Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
[3] Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384(8):705-716. doi:10.1056/NEJMoa2024850
[4] Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21(3):145-161. doi:10.1038/s41568-020-00323-z
[5] Ganatra S, Redd R, Hayek SS, et al. Chimeric Antigen Receptor T-Cell Therapy-Associated Cardiomyopathy in Patients With Refractory or Relapsed Non-Hodgkin Lymphoma. Circulation. 2020;142(17):1687-1690. doi:10.1161/CIRCULATIONAHA.120.048100
[6] Hashmi H, Mirza AS, Darwin A, et al. Venous thromboembolism associated with CD19-directed CAR T-cell therapy in large B-cell lymphoma. Blood Adv. 2020;4(17):4086-4090. doi:10.1182/bloodadvances.2020002060
[7] Goldman A, Maor E, Bomze D, et al. Adverse Cardiovascular and Pulmonary Events Associated With Chimeric Antigen Receptor T-Cell Therapy. J Am Coll Cardiol. 2021;78(18):1800-1813. doi:10.1016/j.jacc.2021.08.044
[8] Barac A, Sharon E. From Detecting Signals to Understanding Cardiovascular Toxicities of Cancer Therapies: All the Light We Could See. J Am Coll Cardiol. 2021;78(18):1814-1816. doi:10.1016/j.jacc.2021.09.008
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



