Frontiers of Nanotechnology in Tumor Immunotherapy
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
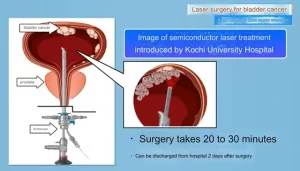
- Kochi University pioneers outpatient bladder cancer treatment using semiconductor lasers
- ASPEN 2024: Nutritional Therapy Strategies for Cancer and Critically Ill Patients
- Which lung cancer patients can benefit from neoadjuvant immunotherapy?
- Heme Iron Absorption: Why Meat Matters for Women’s Iron Needs
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
Frontiers of Nanotechnology in Tumor Immunotherapy
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Frontiers of Nanotechnology in Tumor Immunotherapy.
Preface
At present, cancer is still the second leading cause of death in the world, and cancers that have spread to remote organs account for more than 90% of cancer-related deaths.
Although tumor immunotherapy has shown great potential in prolonging the survival of patients, unfortunately, there is still a lack of effective metastasis therapy because it is difficult to selectively target these small, non-localized distributions on various organs. Tumors.
Nanotechnology has great prospects in improving the immunotherapy effect of patients with metastatic cancer.
Different from traditional cancer immunotherapy, rationally designed nanomaterials can trigger specific tumor-killing effects, thereby improving the contact of immune cells to major metastatic sites such as bone, lung and lymph nodes, optimizing antigen presentation, and inducing a lasting immune response.
Specifically, it can directly reverse the immune status of the primary tumor, use the potential of peripheral immune cells to prevent the formation of the niche before metastasis, and suppress tumor recurrence through postoperative immunotherapy.
In addition, compared with low-molecular-weight immunomodulators, nano-scale immunomodulators have controllable pharmacokinetic behavior.
Due to their unique size effect and the co-carrying capacity generated by the existence of multiple functional domains, they have the ability to achieve synergy The effect enhances the potential for immune activation, which may overcome the obstacles of effective immunotherapy for solid tumors.
Nanotechnology has broad application potential in tumor immunotherapy, especially refractory and relapsed cancers.
Reverse the immune status of the primary tumor
The occurrence of tumor is a process, which is caused by the host immune surveillance deficiency caused by a series of cancer escape mechanisms.
In primary solid tumors, due to the immunosuppressive state of the tumor microenvironment, the immunogenicity of cancer cell neoantigens is too weak to effectively stimulate the immune response.
Nanomaterials provide a new way to overcome this barrier to therapeutic effects.

Change the immunosuppressive tumor microenvironment
The characteristics of the tumor microenvironment play an important role in determining the success of cancer immunotherapy.
In the tumor immunosuppressive microenvironment, a variety of immune and non-immune cell types cause long-term inflammation and local immune suppression, so that malignant cells are not detected and eliminated by the host immune system.
In order to break this situation, nano immunomodulators are designed to directly target the immunosuppressive microenvironment, thereby reactivating the immune system in situ and inhibiting tumor growth.
Macrophages are one of the most abundant immune cell populations in the tumor microenvironment. TAM is mainly M2 phenotype.
Therefore, reverting TAMs from M2 to M1 has become one of the immunotherapy strategies.
Recently, Chen and his colleagues constructed a programmable cell vesicle to combat tumor recurrence and metastasis after surgery. Hybrid cell membrane nanovesicles ( HNV ) can interact with circulating tumor cells ( CTC ) in the vascular cavity and accumulate at the resection site, blocking the CD47-SIRPα interaction, repolarizing TAM from M2 to M1, thereby killing cancer cell.
These nanovesicles can also improve the killing ability of T cells against malignant cells through antigen presentation, and significantly improve the survival rate of malignant melanoma model mice by reducing local recurrence and distal postoperative spread.

In addition to TAM, MDSC also participates in the production of an immunosuppressive microenvironment.
Shuai and colleagues designed a nanoregulator containing MnO2 particles and PI3Kγ inhibitor IPI549 to reduce hypoxia and at the same time down-regulate the expression of immunosuppressive PD-L1 molecules.
At the same time, nanomodulators can activate MDSCs to accelerate the polarization of TAM to the M1 phenotype, and reactivate cytotoxic T cells to prevent tumor cell proliferation.
In addition to affecting TAMs and MDSCs, NK cell activation is another strategy to alleviate the immunosuppressive microenvironment.
Nano-adjuvant immunotherapy using a variety of cytokines, such as IL-2 and IL-12, has been explored to prevent progression and metastasis by activating NK cells.
Irvine and colleagues developed a combined treatment system with agonistic anti-CD137 and IL-2 on the surface of pegylated liposomes.
Through this liposome-based treatment, immune stimulants can quickly accumulate in tumors, thereby inducing effective activation of NK cells and T cells, thereby inhibiting tumor progression at primary tumor sites and lung metastases in mice.
A similar approach to nanomaterials that assist NK cell activation by cytokines has been extensively developed for the treatment of metastatic cancer.
In addition to directly activating NK cells, nanoparticles can also be designed to improve the accumulation of NK cells at the tumor site, thereby enhancing the therapeutic effect.
In general, by reducing the immunosuppressive effect of tumor-related immune cells, reshaping the immune status of the tumor microenvironment provides a feasible way for local and regional tumor immunotherapy.
More importantly, this strategy has great potential to prevent distant tumor metastasis and recurrence through a systemic anti-cancer immune response.
Activate immune cells through ICD
In recent years, studies have shown that traditional local treatment methods, including local hyperthermia, radiotherapy or chemotherapy, not only destroy primary tumor cells, but also induce tumor immunogenic cell death ( ICD ).
However, the immune response stimulated by ICD is usually not enough to cause a systemic effect against metastasis or prevent tumor recurrence.
Therefore, nanomaterials are designed to enhance the immune response to traditional cancer therapies.
Local area heat treatment is the most commonly used method to induce ICD. In a study, Wang and his colleagues used 2D polypyrrole nanosheets with systemic drug delivery and erythrocyte membrane as the NIR-II photothermal sensor to generate a coordinated photothermal and immune response, which is beneficial to prevent metastasis and prolong the survival of mice.
In addition to photothermal therapy, magnetothermal therapy is another feasible method to induce ICD. Liang and colleagues designed a new type of ferromagnetic iron oxide nanoring with eddy current domain, which can mediate gentle magnetic hyperthermia, leading to the expression of calreticulin in 4T1 breast tumor cells and promoting phagocytosis by immune cells Tumor cells.

In addition to local hyperthermia, nano-adjuvant radiotherapy is another treatment strategy that can cause tumor ICD and may inhibit distant metastasis.
In fact, the remote effect of radiotherapy has been observed in some clinical cases.
More formally, it is the radiation-induced bystander effect ( RIBE ), which is triggered by an immune response to dead tumor cells. Through the combination of nano-assisted immunotherapy, RIBE can be significantly expanded.
For example, Liu and colleagues developed a new radioisotope therapy through a combination of 131I-Cat, natural polysaccharide alginate, and synthetic cytosine guanosine phosphate ( CpG ).
After intratumor injection, due to the presence of endogenous Ca2+, polysaccharides quickly form a hydrogel, which fixes 131I-Cat on the tumor site, thereby completely eliminating the primary tumor through low-dose radiotherapy.
Importantly, with the help of immunostimulatory oligonucleotide CpG, the production of tumor-associated antigens ( TAAs ) after local radiotherapy of primary tumors effectively triggers the systemic anti-tumor immune response, combined with checkpoint blocking therapy , Successfully prevented metastasis and recurrence.
These studies help to illustrate the huge potential of tumor ICD combined with nano-adjuvant immunotherapy in preventing tumor growth and metastasis. Combination therapy provides a huge opportunity for the clinical application of nanomaterial-assisted immunotherapy.
Applications for immune cells
Immune cells are located in peripheral immune organs, including lymph nodes, spleen, skin, and vascular system.
They play an important role in generating an immune response stimulated by foreign antigens. Nanotechnology can be applied to immune cells in a variety of ways.
Cancer vaccine against dendritic cells
Cancer vaccines based on dendritic cells have great potential in tumor prevention and treatment, and have been proven to effectively inhibit tumor metastasis and recurrence.
However, DC-based immunotherapy is still limited by insufficient immune response, which makes it difficult to completely eradicate established solid tumors.
Due to the latest advances in nanotechnology, structures such as liposomes, polymer nanoparticles, and inorganic nanoparticles can be loaded with different components, including small molecules, peptides, nucleic acids, and cell membranes.
This allows the antigen and adjuvant to be co-loaded in the nanovaccine, ensuring that these active ingredients are delivered to the same APC at the same time.
In addition, nano-vaccine can also prevent the rapid diffusion of antigens and adjuvants into the blood circulation, and promote their effective accumulation in draining lymph nodes.
Therefore, nanoparticle-based vaccines may be valuable tools to enhance immune response and prevent tumor metastasis.

Zhou et al. constructed an adjuvant/antigen co-delivery nanoplatform by coating PLGA nanoparticles with a phospholipid membrane.
This nano-vaccine can effectively accumulate in lymph nodes and trigger an antigen-specific T cell response, thereby inhibiting the metastasis of B16-OVA melanoma cells, and the number of metastatic nodules is greatly reduced. In another example, by encapsulating the new TLR7/8 bispecific agonist 522NP, an anti-metastatic nanovaccine based on PLGA nanoparticles was developed.
After intravenous injection, 522NPs enters the draining lymph nodes and activates DC, thereby significantly enhancing the subsequent CTL response.
Lung metastatic nodules in OVA+522NP-immunized mice were reduced by about 75% compared with the control group.
Artificial Immune Cells
Artificial APC ( aAPC ) based on micromaterials and nanomaterials aims to inhibit tumors by simulating natural APCs to present antigen signals to T cells and activate them.
In order to achieve the antigen presentation effect, the surface of aAPC should include two parts: MHC peptide complex and costimulatory molecules that can bind to costimulatory receptors and activate T cells.
Compared with natural dendritic cells, aAPC has a relatively clear composition and controllable biological behavior.
In addition, aAPCs can be used for large-scale production, so that ready-made vaccines can be obtained.
Lu et al. developed an aAPC vaccine in which mini-latex beads were assembled with H-2Kb-Ig/pTRP2 dimer complex, anti-CD28 antibody, 4-1BB ligand and CD83 molecule.
In B16 melanoma mouse animal model, intravenous injection of H-2Kb-Ig/pTRP2 aAPCs can stimulate melanoma-specific CTL.
The results showed that lung metastasis was significantly reduced after H-2Kb-Ig/pTRP2 aAPC treatment.
After treatment, only about 36 metastatic nodules appeared in the lung, while about 330 lung metastasis nodules appeared in the control group mice.
In addition, APC mimics are not the only immune cells explored using nanomaterials.
Neutrophils or Tregs also play a key role in the innate immune response to tumors, and nanomaterials have been used to simulate them to inhibit tumor growth.
It can be expected that in future research, new bionic nanomaterials that can simulate more other types of tumor-related immune cells will be developed.
Adoptive T cell therapy
Adoptive T cell therapy is one of the main methods to treat cancer.
The use of nanotechnology can effectively design lymphocytes to express T cell receptors or chimeric antigen receptors, further expanding the successful application of ACT in cancer treatment.
For example, Irvine and colleagues demonstrated that modifying the surface of T cells with cytokines or drug-loaded nanoparticles can significantly improve the efficacy of ACT.
They also used nanogels to load protein drugs onto T cells. This strategy significantly increases the number of T cells present in tumors, thereby improving the safety of ACT treatment.
In addition to T cells, platelets are also used in ACT. Due to their inherent properties, they can accumulate spontaneously in the wound area.
Inspired by this ability, Gu and colleagues bind anti-PD-L1 to the surface of platelets.
These adoptive platelets can successfully target surgical wounds after tumor resection, and release anti-PD through platelet-derived microparticles after platelet activation in situ. -L1.
This strategy has proven to be able to successfully remove residual tumor cells and prevent cancer recurrence.
Niche formation before disturbance transfer
Primary tumors need to change the microenvironment of distant organs to create favorable conditions for CTC, which is called the pre-metastasis niche ( PMN ).
In this phenomenon, the primary tumor cells first secrete soluble components, such as exosomes ( EV ), at the potential metastasis site , and regulate the microenvironment of the region by transferring small nucleic acid fragments to normal cells.
Primary tumor-derived inflammatory factors can simultaneously recruit suppressive immune cells, including MDSCs, TAMs or Tregs, which further secrete chemokines and cytokines to support the formation of PMN.
Therefore, interference with the formation of PMN is an opportunity to prevent tumor cell colonization.
Reshape the molecular composition of PMN
PMN can support the extravasation, anchoring, survival, proliferation and immune evasion of CTC by changing the vascular state of tissues. In addition, PMN also exhibits inflammation and matrix reprogramming, which is also a key process for CTC colonization and survival.
To prevent these processes, researchers have discovered molecules that can reshape PMN components to inhibit metastasis. Lysyl oxidase is an enzyme overexpressed in tumors and PMN, which helps tumor cell colonization by remodeling ECM.
In addition to small molecules, nano-pharmaceutical preparations targeting the molecular components of PMN have also begun to appear. Jiang et al. designed mixed particles of metformin and docosahexaenoic acid as PMN remodeling agents.
These nano-drugs reduced the adhesion between CTC and endothelial cells and reversed the abnormal expression of inflammatory molecules, including PMN.
The fibronectin, matrix metalloproteinase-9 ( MMP-9 ) and S100A9, therefore show the effect of inhibiting metastasis.
Inhibit MDSC
Among the immunosuppressive cells of PMN, MDSC plays a key role in the formation of PMN and can inhibit the activity of CD8+ T cells.
Therefore, inhibiting the recruitment of MDSC is an effective way to prevent metastasis.
Nanotechnology has been used to intervene in the early recruitment of MDSC. Low molecular weight heparin and tocopherol succinate are used to self-assemble into micellar nanoparticles ( LT NPs ).
The former inhibits P-selectin/PSGL-1 mediated extravasation of granulocyte-derived suppressor cells ( g-MDSCs ) through competitive binding, and the latter inhibits the expression of MMP-9 in g-MDSCs through competitive binding.
In addition, the absence of MDSCs in PMN is another way to inhibit metastasis. Ni et al. reported that hafnium DBP (5,15-bis(p-benzoic acid) porphyrin), a nano-scale metal organic compound, combined with αPD-L1 showed excellent anti-tumor activity and anti-tumor activity in an orthotopic breast cancer lung metastasis model Anti-metastatic effect.
Further studies have shown that the anti-metastasis effect comes from the reduction of monocyte MDSC ( mMDSC ) and granulocyte MDSC in the lung , as well as the reduction of mMDSC in the primary tumor.
Block carcinogenic EV
Exosomes are important messengers of primary tumor cells and stromal cells, and can reshape the microenvironment of remote organs.
Interfering with EVs can block the signal transmitted by the primary tumor and may help treatment.
A promising strategy to interfere with exosomes is to directly eliminate exosomes in the circulation. Xie et al. innovatively proposed to drag exosomes into the small intestine through the hepatobiliary metabolic pathway of nanoparticles.
Specifically, they designed an epidermal growth factor receptor targeting aptamer, which is coupled to positively charged mesoporous silica nanoparticles to recognize and bind negatively charged exosomes in the blood.
In vivo experiments show that these nanomaterials effectively increase the distribution of exosomes in the liver and small intestine.
Used in postoperative immunotherapy
In order to solve the problem of recurrence after surgery, various nano immunomodulators have been developed for immunotherapy after surgical resection.
The Gu team developed a nanoformulation that can be sprayed into the tumor resection cavity, which can form an immunotherapeutic fibrin gel in situ after spraying, and block the interaction between CD47 and SIRPα by gradually releasing anti-CD47 antibodies to induce macrophages Cells phagocytize tumor cells.
This nano preparation can effectively inhibit local and distant tumor recurrence after surgery.

Nanomaterial-assisted postoperative immunotherapy has a greater advantage in preventing long-term recurrence of tumors that grow in specific locations, and it is very undesirable to remove additional tissue at these locations, such as brain tumors.
Jiang and colleagues proposed an injectable self-made oligopeptide hydrogel system, which can promote tumor-specific immune response after surgical resection of glioblastoma and effectively prevent the recurrence of brain tumors in mice. The hydrogel serves as a drug library for co-delivery of CXCL10 and tumor homing immune nanomodulator ( THINR ).
After administration in the surgical cavity, the precursor solution forms a hydrogel and is released over time, including mitoxantrone and IDO-targeted small interfering RNA ( siIDO1 ). Mitoxantrone and siIDO1 are decomposed and released in the acidic environment after internalization, and have an immunomodulatory effect on tumor cells, thereby activating circulating T cells and alleviating the immune suppression of Treg.
The activated T cells are then recruited by CXCL10 to the brain to attack the remaining tumor cells.
Compared with systemic administration, local administration of nanomaterials may reduce adverse reactions by limiting organ exposure while increasing the drug concentration in the affected area. Due to the activation of tumor-specific immunity and the formation of long-term immune memory, local area nanomaterials assisted postoperative immunotherapy is a feasible strategy to prevent tumor recurrence after surgical resection.
Summary
Nano immunomodulators can not only effectively eliminate primary tumors, but also have a good inhibitory effect on remote metastasis, and can prevent recurrence.
In addition, nanomaterials can be used as a multifunctional platform to supplement the deficiencies of various immunotherapies.
The emergence of more immunotherapy combinations, artificial immune cells and new nanomaterials will profoundly affect the ability to treat refractory and metastatic cancers.
references:
1. Nanotechnology-enhanced immunotherapy formetastatic cancer. Innovation (NY). 2021 Nov 28; 2(4): 100174.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.