Novartis announces the first clinical data of CD19 CAR-T under the new technology
- A Persistent Crisis: The Looming Specter of Drug Shortages in United States
- Rabies: The fatality rate nearly 100% once symptoms appear
- Human Brain Continues to Grow: Study Shows Increase in Size and Complexity
- CRISPR Genome Editing: From Molecular Principles to Therapeutic Applications
- Metformin Helps Immune System Better Recognize Cancer Cells
- Highlights of Prostate Cancer Research at the 2024 EAU Congress
Novartis announces the first clinical data of CD19 CAR-T under the new technology
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Novartis announces the first clinical data of CD19 CAR-T under the new technology, the treatment preparation time is less than 2 days. Better than Kymriah?
On December 13, Novartis shared the first human test data of CAR-T manufactured by its innovative technology for DLBCL at the annual meeting of the American Society of Hematology (ASH).
The extension of the in vitro T cell culture cycle will deplete the CAR-T final product of the original and stem cell memory T cell subsets that are related to improving the anti-tumor efficacy.
Novartis’ YTB323 is an autologous CD19-targeted CAR-T cell therapy.
Its manufacturing process uses an innovative and simplified process called T-Charge TM to shorten the in vitro culture time to about 24 hours and the time required to manufacture the final product Less than 2 days, eliminating the complexity such as long cultivation cycle.
The improved process retains the initial and stem cell memory T cells in the final product, which is expected to extend the persistence of CAR-T cells, thereby increasing the response rate and duration of response.
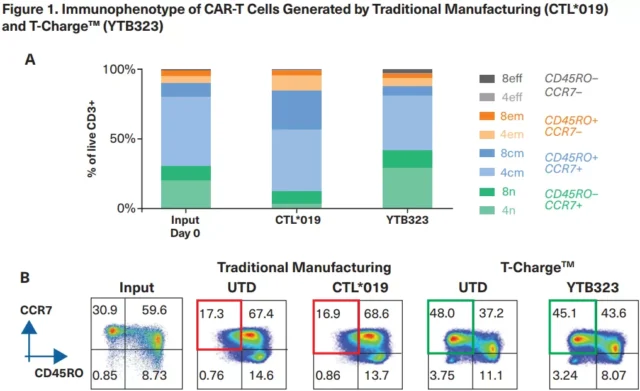
In YTB323, primitive/stem cell memory T cells (CD45RO–/CCR7+) are retained. (Source: ASH official website)
YTB323 has the same CAR transgene as Kymriah. In a small trial, Novartis tested the effect of its therapy at low dose levels in accordance with the standard of conventional CD19 CAR-T therapy, and found that the YTB323 response rate far exceeded Kymriah.
Currently, the Phase I multicenter dose escalation study (NCT03960840) aims to evaluate the safety and preliminary efficacy of YTB323 in the treatment of patients with B-cell malignancies.
Patients received a single dose of YTB323 at 3 different dose levels. Dose level 1 (DL1) is 1–2.5×10 6 CAR+ cells, DL2 is 5–12.5×10 6 CAR+ cells, and DL3 is 25–40×10 6 CAR+ cells.
The primary endpoint is the dose-limiting toxicity (DLT) rate for the first 28 days and the safety of the established recommended phase II dose (RP2D).
Secondary endpoints are cell dynamics, overall response rate (ORR), duration of response, and overall survival rate (OS).
As of April 16, 2021, 15 cases of relapsed/refractory (r/r) DLBCL were treated with YTB323: 4 cases with DL1 level, 10 with DL2 level, and 1 case with DL3 level.
The median age was 65 years; most patients (60%) had previously received 2 treatments, and 4 patients (27%) had received autologous hematopoietic stem cell transplantation (aHSCT).
In terms of safety, among the 15 evaluable patients, 4 cases (27%) were reported with at least one grade 3 adverse event (AE), 6 cases (40%) were reported with at least one grade 4 AE, and 2 cases ( 13%) At least one grade 5 AE.
The most common grade 3/4 adverse events were thrombocytopenia, neutropenia, and decreased neutrophil count. There were 4 cases (27%) of patients with cytokine release syndrome, of which 3 cases (20%) were grade 1/2 and 1 case (7%) was grade 4, which met the DLT standard.
There were 4 deaths in the trial, none of which were related to YTB323 (2 cases of disease progression, 2 cases of sepsis).
In terms of effectiveness, at the DL1 level, the efficacy of 4 patients can be evaluated at the third month, and the ORR and CR rates are both 25% (95%CI, 0.6%-80.6%).
At the DL2 level, the efficacy of 8 patients can be evaluated at the third month, and the ORR and CR rates are both 75% (95% CI, 34.9%-96.8%).
Although the long-term persistence cannot be assessed, during the 3-month follow-up of these 8 patients, 3 of the 8 patients also detected CAR expression by flow cytometry (≥1%).
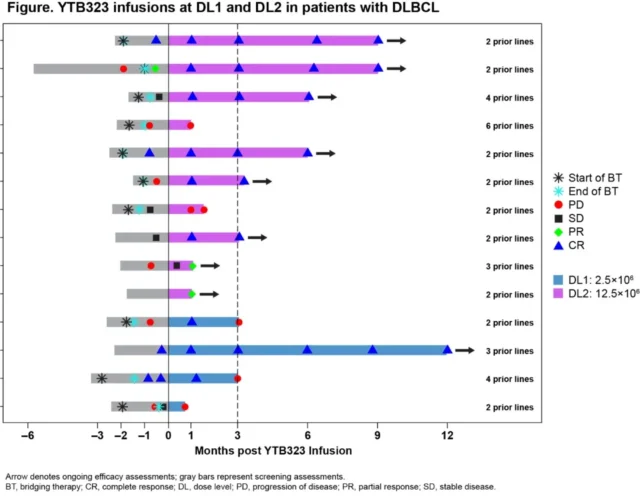
Source: ASH official website
In general, at the DL2 level, YTB323 shows good efficacy and safety.
Current data supports the continued development of YTB323 in patients with r/r DLBCL. A trial of the DL3 level of this therapy is recruiting patients.
The recommended phase II dose is still to be determined.
In addition, Novartis also used the preparation process in the BCMA-targeted CAR-T therapy (PHE885), which is used for multiple myeloma (MM), which is also the target area of Johnson & Johnson and Bristol-Myers Squibb .
Similarly, PHE885 is still in its early stages, and the ASH summary only provides data on 6 MM patients, but the response rate at the second dose level is currently 100%.
Johnson & Johnson has set a high-efficiency standard in the field of BCMA targeted CAR-T, but Dr. Jennifer Brogdon, head of cell therapy research at the Novartis Institute of Biomedical Research, believes that PHE885 has brought some new things to the field.
She said that she has not seen any The product achieves the cell dynamics effect shown by Novartis in MM.
Of course, it is still too early to discuss the durability of these two drug candidates, but because the early data is consistent with Novartis’s expectations for T-Charge, the team has already embarked on more rigorous testing. “We are ready to include them in the authoritative registration trials in 2022.” said Dr. Jeff Legos, Global Head of Oncology and Hematology Development, Novartis.
References:
1# 740 A First-in-Human Study ofYTB323, a Novel, Autologous CD19-Directed CAR-T Cell Therapy Manufactured Usingthe Novel T-ChargeTM platform, for the Treatment of Patients (Pts) withRelapsed/Refractory (r/r) Diffuse Large B-Cell Lymphoma (DLBCL) (Source: ASH official website)
2# 2848 Preservation of T-CellStemness with a Novel Expansionless CAR-T Manufacturing Process, Which ReducesManufacturing Time to Less Than Two Days, Drives Enhanced CAR-T Cell Efficacy (Source: ASH official website)
Novartis announces the first clinical data of CD19 CAR-T under the new technology
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



