Robust clinical data on Roche 3 bispecific antibodies was released!
- A Persistent Crisis: The Looming Specter of Drug Shortages in United States
- Rabies: The fatality rate nearly 100% once symptoms appear
- Human Brain Continues to Grow: Study Shows Increase in Size and Complexity
- CRISPR Genome Editing: From Molecular Principles to Therapeutic Applications
- Metformin Helps Immune System Better Recognize Cancer Cells
- Highlights of Prostate Cancer Research at the 2024 EAU Congress
Robust clinical data of Roche 3 bispecific antiboRobust clinical data on Roche 3 bispecific antibodies was released!-mosunetuzumab, glofitamab, and cevostamab!
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Robust clinical data on Roche 3 bispecific antibodies was released-Mosunetuzumab, Glofitamab, and Cevostamab!
 Structure characteristics of mosunetuzumab
Structure characteristics of mosunetuzumab
Roche’s Genentech recently announced the key clinical data of bispecific antibodies mosunetuzumab (CD20xCD3), glofitamab (CD20xCD3), and cevostamab (FcRH5xCD3) .
The results continue to show that the three bispecific antibodies have shown encouraging efficacy in clinical trials and have good safety.
At present, Roche is continuing to evaluate the above-mentioned bispecific antibodies in an extensive and comprehensive clinical program, as a monotherapy and in combination with other marketed and/or novel therapies, with the aim of providing tailored treatment programs for patients with malignant hematological diseases .
Both mosunetuzumab and glofitamab (formerly known as CD20-TCB) are CD20xCD3 bispecific antibodies that can simultaneously bind to CD20 on the surface of malignant B cells and CD3 on the surface of T cells, bringing T cells close to tumor cells and eliminating tumor cells. Mosunetuzumab and glofitamab are different in structure.
The structure of mosunetuzumab is similar to human natural antibody, but it contains two Fab regions, one of which targets CD20 and the other targets CD3.
Glofitamab has a “2:1” novel structure pattern, containing two Fab regions targeting CD20, and one Fab region targeting CD3.
This dual targeting effect can activate and redirect the patient’s existing endogenous T cells, bind to and eliminate these malignant B cells by releasing toxic proteins into the target B cells.
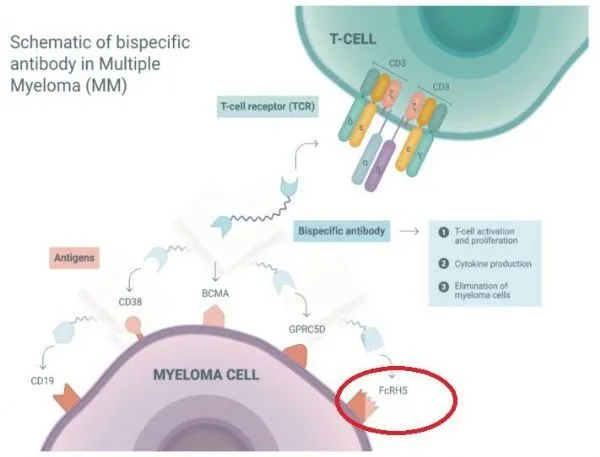
Cevostamab is a first-in-class FcRH5xCD3 T cell binding bispecific antibody that can simultaneously bind FcRH5 on myeloma cells and CD3 on the surface of T cells, bringing T cells close to tumor cells and eliminating tumor cells.
FcRH5 is a unique and differentiated target, expressed on almost 100% of myeloma cells. The structure of cevostamab is similar to human natural antibody, but contains two Fab regions, one of which targets FcRH5 and the other Fab region targets CD3.
Such dual targeting can be activated and reboot the patient prior endogenous T cells, myeloma cells expressing binding FcRH5 by releasing cytotoxic proteins into the target myeloma cells, to eliminate these targets malignant tumor cells.
Currently, cevostamab is being developed to treat patients with relapsed or refractory multiple myeloma (R/R MM).
 FcRH5xCD3 (picture from literature: PMID 32659909)
FcRH5xCD3 (picture from literature: PMID 32659909)
- Preliminary results from the phase 1b CO41942 study show that the mosunetuzumab+lenalidomide combination regimen has an initial effect in patients with relapsed or refractory follicular lymphoma (R/R FL) who have received at least one treatment in the past Encouraging, safety is tolerable.
- The results from the GO40516 study of Phase 1b/2 indicate that mosunetuzumab+Polivy ( Polatuzumab vedotin-piiq) combination regimen has good efficacy and safety: the objective response rate (ORR) reached 65.0%, and the complete response rate (CR) reached 48.3% . Cytokine release syndrome (CRS) occurs in 18% of patients, and all events occur in the first cycle, which is grade 1-2.
- Phase 1/2b NP30179 dose escalation study evaluated glofitamab as a monotherapy, glofitamab+Gazyva (obinutuzumab) combination regimen in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (R/R B-NHL) In the efficacy and safety. The results show that R/R FL and R/R mantle cell lymphoma (MCL) have an encouraging effect. Preliminary results in R/R FL patients who have previously received multiple regimens showed that all treatment groups (including high-risk subgroups) showed high remission rates: the ORR of the glofitamab single-agent group was 81.0%, and the glofitamab+Gazyva The ORR of the combination treatment group was 100% . For R/R MCL patients who were previously treated with glofitamab after receiving Gazyva treatment, the ORR was 81.0% . In the study, the most common adverse event was CRS, and most of the events were of low grade (grade 1-2).
- The results of the phase 1b/2 NP39488 study show that the initial efficacy of the glofitamab+Polivy combination regimen in patients with difficult-to-treat R/R diffuse large B-cell lymphoma (DLBCL) is encouraging, and the safety is tolerable . The median follow-up was 3.2 months (95%CI: 1.4-3.5), the ORR was 73.0%, the CR was 51.5%, and the duration of remission was ≥6 months. No CRS events of grade 3 or higher were observed, and the safety of combination medication was consistent with that of single medication.
- Data from the Phase 1 GO39775 Dose Escalation and Expansion Study showed that in patients with R/R multiple myeloma (MM) who had previously received multiple regimens, the first FcRH5xCD3 bispecific antibody cevostamab induced clinically significant, Targeted dose-dependent increase in ORR without increasing the rate of CRS. The ORR of the 160 mg dose group was 54.5% . The results of 2-step escalation administration show that, compared with single-step escalation administration, this method helps to alleviate CRS and may improve safety.
Reference:
Genentech Presents Pivotal Data at ASH 2021 for Novel Cancer Immunotherapy Mosunetuzumab
Robust clinical data on Roche 3 bispecific antibodies was released!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



