The role of inducible costimulatory molecule ICOS in the differentiation of CD8+ T lymphocytes
- A Persistent Crisis: The Looming Specter of Drug Shortages in United States
- Rabies: The fatality rate nearly 100% once symptoms appear
- Human Brain Continues to Grow: Study Shows Increase in Size and Complexity
- CRISPR Genome Editing: From Molecular Principles to Therapeutic Applications
- Metformin Helps Immune System Better Recognize Cancer Cells
- Highlights of Prostate Cancer Research at the 2024 EAU Congress
Immunity: The role of inducible costimulatory molecule ICOS in the differentiation of CD8+ T lymphocytes
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Immunity: The role of inducible costimulatory molecule ICOS in the differentiation of CD8+ T lymphocytes.
Immune memory is one of the important mechanisms used by the organism’s adaptive immune system to resist the re-invasion of pathogens.
Compared to naive T cells, memory T cells (Memory T cells) after re-encounter the same antigen can be faster and more intense reaction in order to effectively control the infection or disease process, which is one of the important underlying mechanism of vaccine.
Recent studies have shown that memory T cells are highly heterogeneous and consist of cell subgroups with different cyclic migration, function and lifespan characteristics (Jameson and Masopust, 2018) .
From the perspective of circulation and migration, memory T cells can be divided into circulating cells and resident cells:
- Circulating cells include central memory T cells ( Tcm ) and effector memory T cells ( Tem ) , which can pass through blood and lymph in various tissues and organs of the body Circulate continuously;
- Correspondingly, tissue-resident memory T cells ( Trm ) reside in non-lymphoid tissues under normal physiological conditions and do not exchange with circulating cells in the blood.
Due to its special location advantages, Trm can play the first-line rapid barrier protection function against the infection of bacteria or tumors in the tissues where it resides (Masopust and Soerens, 2019) .
Therefore, the research on regulating the differentiation and maintenance of Trm fate has considerable significance.
T cell co-stimulatory molecule (Co-stimulatory molecule) signals can work with T cell receptors (TCR) , cytokines in the environment, etc., to affect the differentiation and fate selection of initial T cells.
Although there are many substantive research advances on cytokines and transcription factors that promote Trm production, whether there are specific costimulatory molecules and whether the interaction between cells in non-lymphoid tissues can induce and determine the differentiation of tissue resident populations Still unknown.
In response to this issue, on December 20, 2021, the Stephen Jameson’s laboratory at University of Minnesota published a research paper in Immunity, entitled Engagement of ICOS in tissues promotes establishment of CD8+ tissue-resident memory T cells .
The research article on T cells revealed that in a mouse acute infection model, the inducible costimulatory molecule ICOS can specifically mediate the efficient maturation and differentiation of CD8+ tissue-resident memory T cells in non-lymphoid tissues.
The inducible costimulatory molecule ICOS has a clear role in the differentiation of CD4+ helper T cells, and it is essential for the differentiation of follicular helper cells (Tfh) in the germinal center response .
However, the role of ICOS in the differentiation of CD8+ cytotoxic T cells remains unclear.
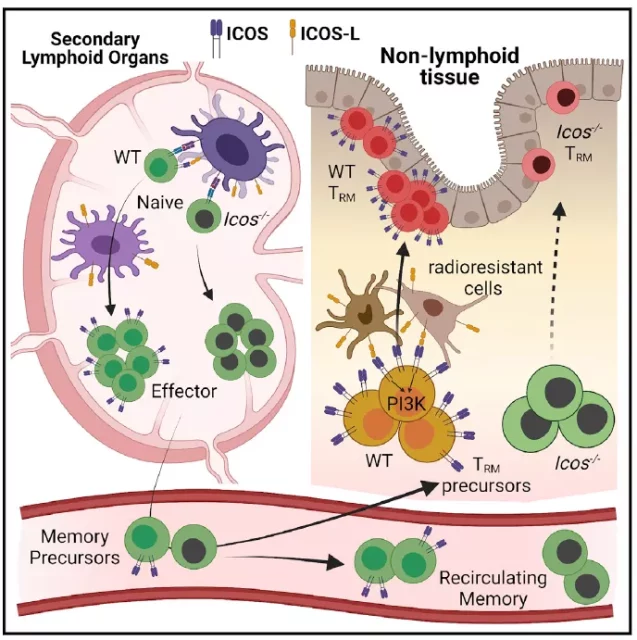
First of all, through different viral or bacterial acute infection models, the researchers found that the number of CD8+ T cells deficient in ICOS compared with wild-type, there is no difference in the peripheral circulating memory T cells, but the resident in non-lymphoid tissues However, the memory cells exhibited a significant lack of specificity.
This finding is mutually corroborated by the high expression of the ICOS gene as a CD8+ resident cell in the previous report.
Through the study of different time points after infection, it is further found that the absence or blockade of ICOS does not affect the initial activation of CD8+ T cells or the expansion of effector cells, and it can migrate to non-lymphoid tissues as effectively as wild-type at the initial stage of infection.
However, defects appear in the process of further differentiation and maturation in the subsequent tissues. In contrast, blocking the ICOS signal of mature CD8+ resident memory cells will not affect their long-term maintenance.
In order to further determine the time and mechanism of the effect of ICOS on CD8+ resident memory cells, the researchers used adoptive transfer to show whether CD8+ T cells were co-stimulated by ICOS during the activation and proliferation stage in the lymphatic tissue at the initial stage of infection.
The signal has no effect on the subsequent maturation and differentiation of Trm in normal non-lymphoid tissues; on the contrary, adoptive transfer of CD8+ effector T cells that normally receive the ICOS costimulatory signal to ICOS-L-deficient mice will have no effect on it.
The differentiation of Trm in lymphoid tissues is hindered. Since the ligand ICOS-L of ICOS is widely expressed in various antigen-presenting cells and non-lymphoid tissue stromal cells and epithelial cells, it is difficult to determine the source of the ICOS costimulatory signal that mediates CD8+ Trm.
Researchers through bone marrow chimera studies have shown that: unlike CD4+ Tfh, which uses B cells in lymphoid organs as the source of ICOS-L, the ICOS-L costimulatory signal of CD8+ Trm originates from non-lymphatic tissues that do not respond to radiation.
Sensitive cell populations-including cell populations derived from non-hematopoietic stem cells or antigen-presenting cells residing in tissues.
Finally, through single-cell sequencing to compare wild-type and ICOS-deficient CD8+ T cells in the early stage of infection and mature memory stage, the researchers proved from the transcriptome that the CD8+ tissue-resident cells lacking ICOS are very different from wild-type CD8+ T cells.
This reflects that ICOS, as a costimulatory signal in the tissue, mainly mediates the efficiency of the differentiation and maturation of CD8+ resident cells without affecting its quality.
Further molecular mechanism studies have shown that the PI3K signal mediated by ICOS and its downstream transcription factor KLF2 play an important role in the tissue residence of CD8+ T cells.
Summary
this study shows the role of the classical costimulatory molecule ICOS in the differentiation of CD8+ T lymphocytes-it can specifically and efficiently induce the production of resident memory cells in the tissue without affecting peripheral circulating memory cells.
In addition, ICOS costimulatory signals mainly act after CD8+ T lymphocytes migrate to non-lymphoid tissues, and are mediated by local radiation-insensitive cell populations.
This is the role of the tissue microenvironment in the differentiation and maturation of tissue-resident memory cells.
New avenues have been opened up. Due to the effectiveness of tissue-resident memory cells for vaccines and their high similarity with tumor infiltrating cells, the target of ICOS/ICOS-L costimulatory signals can become a new idea for the treatment of related diseases.
Original link:
https://doi.org/10.1016/j.immuni.2021.11.017
The role of inducible costimulatory molecule ICOS in the differentiation of CD8+ T lymphocytes
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.