Japanese scientists developed a vaccine that can eliminate senescent cells
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Japanese scientists developed a vaccine that can eliminate senescent cells
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Japanese scientists developed a vaccine that can eliminate senescent cells.
Anti-aging vaccine is born! Japanese scientists have developed a vaccine that can target to eliminate senescent cells, which can effectively delay the aging process of mice.
People’s sigh for the short life and the pursuit of immortality have been accompanied by thousands of years of civilization.
The elixir, alchemy, and vampire legends that emerged from this have all become classics that cannot be forgotten in legends.
As our understanding of the world continues to deepen, scientists have turned their attention to how to rely on science to extend life.
Recently, the “Nature·Aging” magazine published the latest masterpiece by Tohru Minamino and others.
The study found a new anti-aging vaccine that can eliminate senescent cells in the body by targeting, which can improve aging longer and more safely.
The metabolic abnormality brought about reduces atherosclerotic plaques, delays the normal aging process, and prolongs the average life span of premature aging mice by 4 weeks [1].
Before opening the article, I believe that many people are like me and don’t know what stage the current anti-aging treatment field has reached. Let’s take the time to chat about the Senolytic treatment, which is now in full swing.
Senolytic, a combination of the two roots of senescence seno and destruction lytic, originated from a kind of thinking to eliminate senescent cells.
Through research on the aging process, people have found that aging is due to the appearance of senescent cells in various systems.
These cells not only lack the physiological functions of healthy cells, but also transmit “bad” messages and drive the entire organ to a downward slope .
Then we selectively Eliminating this part of the cells may prevent the occurrence of senescence.
This concept was originally created because scientists discovered that the leukemia chemotherapy drug dasatinib and a plant extract quercetin can selectively eliminate senescent cells in the body, so the two drugs were combined orally used to delay Aging, this is the famous “D+Q” therapy [2], which has now entered the second clinical stage.
Although the medicine has been found, people still don’t understand the clearing mechanism of senescent cells very well. If you know it but don’t know the reason, it is easy to go wrong.
What’s more, friends who know chemotherapeutics know that chemotherapeutics can damage normal cell and organ function, so we need to find safer and more specific drugs.
If you were a researcher, how would you think?
First of all, the reason why senescent cells can be specifically eliminated must be because they have their own special markers.
So the scientists screened out 10 transmembrane proteins that are highly expressed in senescent cells through the GEO database and the KEGG database, and finally set their sights on a protein called Gpnmb.
Why choose transmembrane protein? Because their next plan is to develop a vaccine for this protein, so that immune cells can kill all the cells expressing the Gpnmb protein for us .
The key for immune cells to play the role of ADCC is to recognize antigens on the surface of abnormal cells, so it is necessary to select transmembrane proteins to activate the immune response.
As our loyal and reliable thugs, the immune cells in our body are specific and efficient, and it can solve the two problems of low specificity and low efficiency in the targeted elimination of senescent cells.
In order to prove the universal applicability of the vaccine, the researchers chose four different models: atherosclerosis, high-fat diet, progeria, and naturally aging mice.
And using these models to prove that the expression of Gpnmb protein in senescent cells is significantly increased, and knocking out the GPNMB gene can reduce the burden of disease in disease model mice.
With these evidences, vaccines can be developed~
The researchers selected part of the extracellular segment of the Gpnmb protein as an antigen to make a vaccine, and injected it subcutaneously into mice, successfully inducing ADCC (antibody-dependent cell-mediated cytotoxicity) against senescent cells in the body.
The first step of the vaccine has been successful. Next, it is necessary to verify whether this targeting effect can alleviate aging.
The researchers selected three entry points:
First, abnormal metabolism .
One of the notable features of senescent cells is the increased expression of β-galactosidase, which is more pronounced in mice on a high-fat diet.
Through the luciferase reporter system, the researchers found that 90% of cells with high Gpnmb protein expression also highly express β-galactosidase, and vaccine injection can significantly eliminate this cell , while for low expression of Gpnmb protein but high expression The β-galactosidase cell has no clearing effect.
When NK cells and CD8+ T cells were suppressed, the clearance effect of the vaccine also disappeared, indicating that the clearance effect of the vaccine was dependent on these two cells.
Second, senile diseases .
Atherosclerosis is a well-known disease of the elderly, and its incidence increases with age.
The researchers used APOE knockout mice, supplemented with a high-fat diet as a mouse model of atherosclerosis, and found that after Gpnmb vaccine treatment, the area of atherosclerotic plaques in the mice was significantly reduced , and the cell flow cytometry results also showed β in vivo -The number of galactosidase positive cells is reduced.
Third, natural aging .
Natural aging is the inevitable end of life. Even if there is no disease, our body functions will inevitably decrease. So can aging vaccines change this natural law?
The researchers vaccinated 50-week-old middle-aged wild-type mice and evaluated their exercise capacity at 70 weeks. They found that compared with the control group, the average speed and total distance of the vaccine-injected mice were both Significantly improved .
In addition, vaccine injection of premature aging mice knocked out by Zmpste24 can significantly prolong the survival life of premature aging mice .
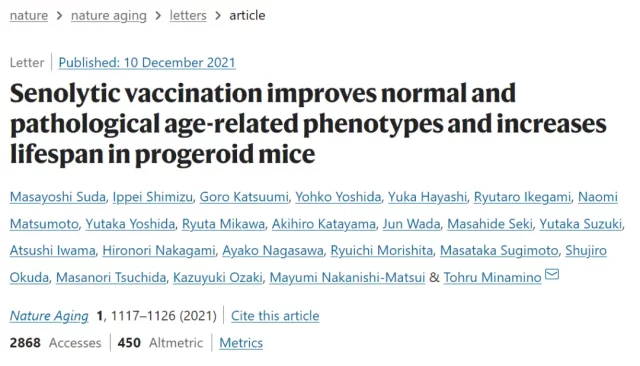
 The picture shows the reduction of Gpnmb positive cells (a) and the improvement of metabolic abnormalities (d, f, g) after vaccine injection
The picture shows the reduction of Gpnmb positive cells (a) and the improvement of metabolic abnormalities (d, f, g) after vaccine injection
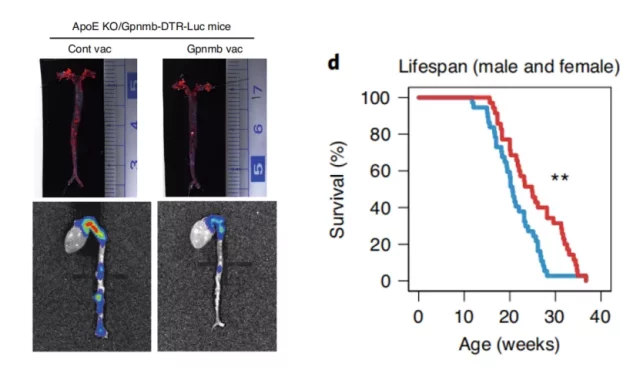
The picture shows that Gpnmb vaccine improves atherosclerosis (left) and prolongs the lifespan of premature aging mice (right)
Finally, the researchers showed us the efficacy of their vaccine compared with other drugs.
As mentioned earlier, “D+Q” is currently the most commonly accepted anti-aging therapy, and another drug that has been studied more is Navitoclax (Bcl-2 inhibitor).
This study compared the Gpnmb vaccine with these two drugs and found that the Gpnmb vaccine has a longer-lasting effect and causes fewer side effects such as anemia .
Along with major breakthroughs, there are often more unsolved mysteries. It has been found that Gpnmb plays a role in the process of osteogenesis and can mediate the adhesion of dendritic cells.
Does it have other functions in the body? Why do senescent cells highly express Gpnmb, and are there any protective and message-transmitting effects?
In addition to removing cells with high Gpnmb expression, the vaccine will inevitably also remove some cells with low Gpnmb expression.
Will there be side effects that we have not yet discovered?
In general, this research puts an important link in the aging puzzle. It also opened up new horizons for the research of anti-aging.
Compared with previous studies, vaccines against senescent cells are not only more stable but also safer, and are likely to be the mainstream direction of Senolytic therapy in the future.
Personally, every time I lament the magic of anti-aging drugs, I still have conservative doubts.
In the long evolution of human beings, many genes that are not suitable for survival have been discarded, and genes that are more conducive to development have been left behind, which constitutes our delicate and coordinated organism.
Will it really leave “useless” genes in the genome? Are there really types of cells in our body that can be eliminated?
Immortality is the long-term pursuit and good wish of mankind, but we are still far away from it.
We still have to bloom the greatest value in our limited life. As for where the aging vaccine will go, let us wait and see!
Reference:
[1] Suda, M., Shimizu, I., Katsuumi, G. et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat Aging (2021). https://doi.org/10.1038/s43587-021-00151-2
[2] Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973-977. doi:10.1111/acel.12458
Japanese scientists developed a vaccine that can eliminate senescent cells
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



