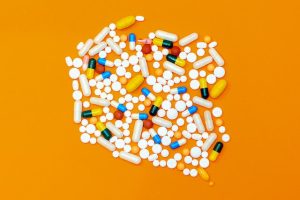
Prospects for the first batch of COVID-19 oral drugs on the market in 2022
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
- Study shows regular seafood consumption may increase risk of exposure to PFAS
- A Persistent Crisis: The Looming Specter of Drug Shortages in United States
- Rabies: The fatality rate nearly 100% once symptoms appear
- Human Brain Continues to Grow: Study Shows Increase in Size and Complexity
- CRISPR Genome Editing: From Molecular Principles to Therapeutic Applications
Prospects for the first batch of COVID-19 oral drugs on the market in 2022
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Prospects for the first batch of COVID-19 oral drugs on the market in 2022.
At the beginning of 2022, the US Food and Drug Administration (FDA) has considered approving two oral anti-coronavirus (COVID-19) virus drugs, Pfizer’s paxlovid and Merck & Co. The launch of molnupiravir, an oral drug, will greatly improve the treatment plan for patients after infection.
What impact will this have on the trend of the epidemic in 2022, and whether it will be affected by the potential mutation of the virus?
These are all countries around the world expected to gradually subside in 2022. The important considerations of, let’s discuss these two molecules with you today.
The COVID-19 virus has been raging around the world for more than 2 years, and major pharmaceutical companies and research units have been committed to developing their treatment options.
With the launch of many vaccines in 2019, their spread has been slowed down to a certain extent.
Due to the emergence of various mutant strains of COVID-19, vaccines have reduced their effects to varying degrees, failing to fundamentally reduce their global prevalence and damage. Small molecule drugs have always been the main treatment for various diseases.
At the beginning of 2022, Pfizer and Merck demonstrated their leadership in the development of innovative drugs in the face of this pandemic crisis.
At an unprecedented rate of development, In less than 20 months, it has pushed its related oral drug molecules into the clinical approval stage, and a convenient treatment plan for small molecule drugs for COVID-19 will soon be launched .
Preliminary clinical data show that these two drugs are superior to Gilead’s remdesivir (remdesivir, an intravenous antiviral drug for inpatients), and to varying degrees can be used as oral antiviral drugs for non-inpatients It brings new hope for the fundamental cessation of the global COVID-19 anti-virus in 2022.
According to existing research and development experience, oral antiviral drugs have several significant potential advantages:
(1) It is cheaper and easier to use than antibodies that have been approved for use in non-hospital patients ;
(2) It may not be resistant to mutations caused by drug resistance. Too sensitive . As we all know, antibodies target the Spike protein used when the sars-cov-2 virus enters human cells. From previous viral studies, the Spike protein is more prone to mutation, which is the weaker part of the virus replication process. For example, the recently discovered Omicron has more than 30 mutations in Spike, but only one mutation in each of the proteins targeted by paxlovid and molnupiravir;
(3) It can complement existing vaccines or inpatient treatments for prevention COVID-19-related deaths provide unprecedented opportunities.
1. Research engine and target selection
After the COVID-19 outbreak, as soon as the SARS-CoV-2 genome was published, researchers from various countries began to analyze the weakness of the 29 proteins contained in the virus.
After combining the research of other viral diseases, the researchers began to focus on the replication mechanism of the virus.

Pfizer chose the main protease (Mpro) of the virus. In the process of virus replication, SARS-CoV-2 will synthesize long polypeptides, which must be cleaved by Mpro into its constituent viral proteins. Inhibition of Mpro prevents the virus from producing the proteins needed for its replication.
Rolf Hilgenfeld of the University of Lübeck in Germany said that Mpro is a good target because it is the Achilles heel of this virus.
Pfizer researchers started designing from an existing lead molecule ( Review of the development of Pfizer’s oral anti-coronavirus drug Paxlovid ).
After rapid optimization, the covalent molecule PF-07321332, which inhibits Mpro enzyme activity, was obtained, and ritonavir (ritonavir, A HIV drug that inhibits cytochrome p450 and slows down the metabolism of protease inhibitors) combined to form paxlovid, which moved from a laboratory idea to a submission to the FDA in just 20 months.
In March 2021, Pfizer started the phase I clinical trial of paxlovid, and started the phase II/III clinical trial in July, which will end in early 2022. An interim analysis of 1,219 patients found that treatment with paxlovid within 3 days after the onset of symptoms reduced hospitalization or mortality by 89%.
Mikael Dolsten, chief scientific officer of Pfizer, said: “This is a fast process. In the normal small molecule project development process, it will take 8-10 years.” In addition to Pfizer, other SARS-CoV targets Mpro. -2 compounds are also under development.
Like Pfizer’s compounds, most of the compounds are peptides that mimic the cleavage of Mpro and are covalently bound to the active site to inhibit the enzymatic activity of Mpro.
Merck’s research focuses on viral RNA replicase (RNA-dependent RNA polymerase, RdRp) . Viral RdRp is an enzyme that synthesizes RNA, which can be converted into viral proteins and can replicate itself.
Its structure is relatively conservative in different virus categories, and its function provides more opportunities for broad-spectrum antiviral drugs. Gilead’s remdesivir is also an RdRp inhibitor.
It took advantage of this broad-spectrum potential and entered the Ebola virus clinical trial for the first time in 2015, but it failed, and it was not approved for use outside the United States in the treatment of COVID-19.
It is important to point out that not all RdRp inhibitors work in the same way.
In most cases (including remdesivir) the virus will incorporate the drug into the extended RNA, thereby stopping the elongation process; molnupiravir has a different mechanism.
When it is integrated into the viral RNA, the elongation does not stop. Instead, the virus reuses RNA containing molnupirvir as a template strand, and merges the wrong bases into the new viral RNA when it encounters molnupirvir again.
Mutations accumulate in the circulation, leading to “wrong mutations” and virus death.
molnupiravir itself originated in the George Painter Laboratory of Emory University.
In 2013, Painter was developing a drug for the treatment of Venezuelan equine encephalitis virus (VEEV), hoping to target RdRp to develop a broadly acting rna-encoded antiviral drug.
He chose to study nucleoside analogues as chemical backbones because they are usually effective, have high drug resistance, and can usually be taken orally.
With the onset of COVID-19, Painter et al. found that in preclinical models, molnupiravir has extensive antiviral activity against SARS-CoV-2, MERS-CoV, SARS-CoV-1 and influenza. ) Licensed the drug to Ridgeback Biotherapeutics.
Two months later, Merck obtained the exclusive global development and commercialization rights for the drug. It entered Phase I trials in April 2020, and Phase II/III MOVe began in October 2020. -OUT trial, and submitted the interim results of the EUA trial to the FDA in October 2021.
Interim results of molnupiravir on 1,433 patients showed that when the drug was used within 5 days of the onset of symptoms, the hospitalization rate or mortality was reduced by 30%, but in the second half of the trial, molnupiravir did not show further advantages.
At the FDA’s advisory committee meeting on the drug, experts voted for approval with a narrow margin.
However, 10 panelists believe that the benefits of this drug do not justify the risks. Because molnupiravir induces errors in viral RNA, in theory it may accelerate the evolution of this virus.
This risk is further increased in immunocompromised patients. In addition, molnupiravir may be incorporated into human DNA, causing mutations in rapidly dividing human tissues, including fetuses .
David Eastmond, a member of the FDA advisory committee, suggested that the FDA should not approve pregnant women to take the drug.
2. Antiviral treatment that requires early intervention
For non-hospital patients, including vaccinated and unvaccinated patients, both drugs are available in outpatient clinics after approval, and the cost of each course of treatment is about US$530 to US$700. The US government has pledged to purchase 10 million courses of Pfizer and 1.7 million courses of Merck.
However, for more severely ill hospitalized patients, these drugs may be less effective, and the earlier the treatment, the better.
Once an infected patient is seriously ill and needs to be hospitalized, all current research evidence shows that most diseases are no longer caused by virus replication, and the inflammatory response will dominate .
Therefore, after treating only about 300 patients, Merck stopped conducting molnupiravir trials in hospitalized patients.
The need for early treatment of the virus may also partly explain why Roche’s use of oseltamivir to treat influenza is not obvious.
The flu patient felt bad for a few days, then went to the doctor and prescribed oseltamivir, but it was too late by then. Early intervention in the first 3-7 days depends on the availability of early detection.
Therefore, the rapid detection of COVID-19 provides an important help for the use of oral antiviral drugs.
3. Facing the future
COVID-19 at the start of a pandemic, British clinical trials expert Horby presided looking directly into the screening phase III clinical antiviral drugs, including possible remdesivir FDA and other new and old drugs used, were not successful, these molecules on the virus In terms of the effect is too weak, he pointed out that in COVID-19, there is no confidence in the success of using old drugs and new drugs.
New antiviral drugs are definitely needed . The community needs to fully evaluate these new drugs in large-scale trials.
Judging from the conservativeness of the target, molnupiravir will become a drug for a broad-spectrum virus, play a certain role in ending this pandemic, and may play an important role in zoonotic diseases spread by other coronaviruses in the future.
Due to the large structural differences between the Mpro of different viruses, paxlovid is more active, but its use may be more limited to SARS-CoV-2.
However, the Mpro inhibitor discovered by Pfizer is designed and optimized on the basis of candidate antiviral drugs with similar targets, which can also provide a good research idea for other antiviral drugs in the future.
references
1. Gajjar ND, Dhameliya TM, Shah GB. In search of RdRp and Mpro inhibitors against SARS CoV-2: Molecular docking, molecular dynamic simulations and ADMET analysis. J Mol Struct. 2021;1239:130488. doi:10.1016/j.molstruc.2021.130488.
2. Cully M. A tale of two antiviral targets – and the COVID-19 drugs that bind them. Nat Rev Drug Discov. 2021 Dec 2. doi: 10.1038/d41573-021-00202-8. Epub ahead of print. PMID: 34857884.
Prospects for the first batch of COVID-19 oral drugs on the market in 2022
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.