Residual tumor burden after neoadjuvant chemotherapy for breast cancer
- Rabies: The fatality rate nearly 100% once symptoms appear
- Human Brain Continues to Grow: Study Shows Increase in Size and Complexity
- CRISPR Genome Editing: From Molecular Principles to Therapeutic Applications
- Metformin Helps Immune System Better Recognize Cancer Cells
- Highlights of Prostate Cancer Research at the 2024 EAU Congress
- High Blood Sugar and Lipids Linked to Mental Health Risks
Residual tumor burden after neoadjuvant chemotherapy for breast cancer
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Residual tumor burden after neoadjuvant chemotherapy for breast cancer.
The Lancet Oncology: Must pay attention to this important indicator for the prognosis of neoadjuvant chemotherapy for breast cancer.
The incidence of breast cancer has surpassed that of lung cancer, becoming the most common malignant tumor that harms humans.
In the treatment of locally advanced breast cancer patients, neoadjuvant chemotherapy can reduce tumor volume and reduce the positive rate of lymph nodes, thereby increasing the success rate of surgical resection [1].
After the patient receives neoadjuvant chemotherapy, if the pathological examination of the surgical specimen shows that the tumor cells have completely disappeared, that is, the pathological complete remission (pCR), the survival time of this type of patient is significantly prolonged [2].
The pCR ratio of different neoadjuvant treatment programs can be used as a sign of the effectiveness of their programs.
But not all breast cancer patients receive neoadjuvant therapy, the tumor cells can completely disappear. pCR is a binary value .
Patients either achieved pCR or did not achieve pCR. Compared with patients who did not achieve pCR, the prognosis was significantly improved.
As for breast cancer patients who have not reached pCR, some patients have more residual tumor cells in the lesion, and some have less residual tumor cells, and the prognosis is not exactly the same .
Residual tumor burden (RCB) is a continuous value that can be used to assess how much tumor remains in breast cancer patients after receiving neoadjuvant therapy .
Some single-center analysis studies have confirmed that RCB can predict the prognosis of patients very well, but these single-center studies usually have small samples, so it is difficult to obtain accurate prediction power.
In large-scale populations and in various breast cancer subgroups, the predictive power of RCB for prognosis is unclear.
In response to this problem, researchers at the University of California, San Francisco , recently published a multi-center retrospective study in the journal The Lancet Oncology [3].
This study found that for every 1 unit increase in the RCB score, the risk of recurrence, metastasis, or death in all breast cancer patients increased by 82% (HR: 1.82) .
Therefore, researchers believe that RCB scores and classifications in different clinical research centers and in different breast cancer subgroups can independently predict the long-term survival of breast cancer patients.
Therefore, it is recommended that RCB be included in the criteria for breast cancer surgery after neoadjuvant chemotherapy. Pathology report .
▲ Screenshot of the paper’s homepage
Next, let’s take a look at how this research is carried out.
This study aggregated 12 independent breast cancer research cohorts from clinical centers in the United States and Europe.
Patients with stage I-III who received neoadjuvant chemotherapy + surgery ± corresponding adjuvant therapy were included.
The primary endpoint of the study is event-free survival (EFS), and the secondary endpoint is remote recurrence-free survival (DRFS).
The RCB score is evaluated and classified by breast cancer pathologists in each treatment center according to standard protocols.
The RCB classification is divided into 4 levels (0, 1, 2, 3). Using the mixed-effect Cox model, the RCB score and grading were analyzed and the relationship with patients’ EFS and DRFS was analyzed.
And according to the patient’s estrogen and progesterone receptor levels, HER2 expression level and other baseline factors for subgroup analysis.
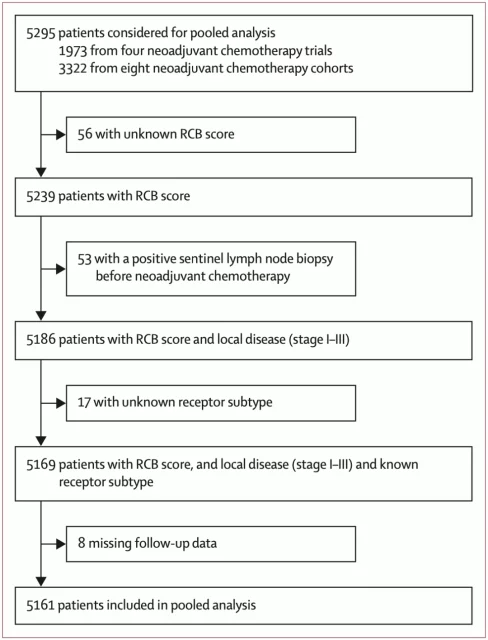
The study finally included 5,616 breast cancer patients , with a median age of 49 years, 1774 (34.4%) patients with triple-negative breast cancer, 1430 (27.7%) patients with HER2- positive breast cancer, and 1957 (37.9%) patients The patient has hormone receptor positive + HER 2 negative breast cancer.
After all patients received neoadjuvant therapy + surgery, the results of RCB analysis of surgical specimens showed that 1676 (32.5%) patients were RCB-0 (pCR), 662 (12.8%) patients were RCB-1, 2017 (39.1%) ) Patients were rated RCB-2, and 806 (15.6%) patients were rated RCB-3.
The results of the univariate correlation analysis between RCB and EFS and DRFS showed that in the entire population, for every increase in the RCB score, the risk of recurrence, metastasis or death increased by 82% (HR: 1.82), and the risk of distant metastasis or death An increase of 86% (HR: 1.86) .
In the corrected multivariate analysis, RCB is still significantly correlated with EFS and DRFS, and HR is 1.69 and 1.75, respectively. The logarithmic value of RCB and risk rate presents a linear positive correlation.
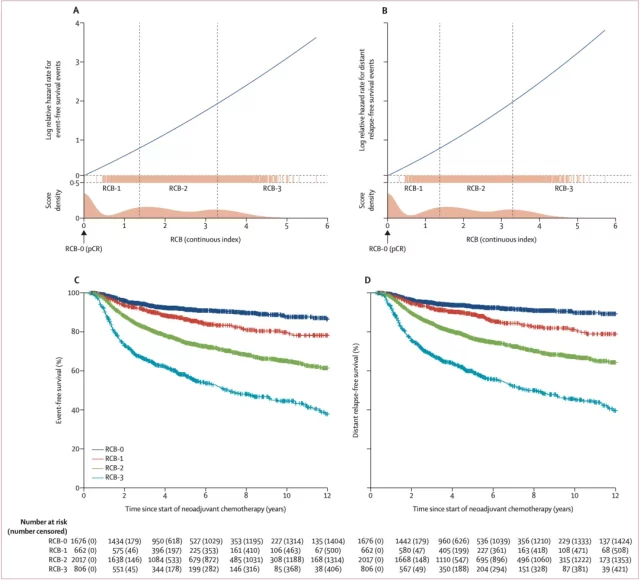
▲ RCB score and EFS, DRFS risk rate logarithmic value of the correlation diagram (A and B);
And EFS and DRFS (C and D) of breast cancer patients with different RCB grades
For each subgroup with different RCB grades, the survival curves can be well separated, which means that RCB grades can predict the prognosis of breast cancer patients well.
Breast cancer patients with different RCB grades have significantly different EFS and DRFS.
The higher the RCB, the lower the EFS and DRFS. RCB=0 grade has the best prognosis, RCB=3 grade has the worst prognosis. During the 12-year follow-up period after neoadjuvant therapy, only the EFS rate and DRFs rate of patients with RCB=3 grade dropped below 50%, that is, more than half of the patients with RCB=3 grade had tumor recurrence, metastasis or death, etc. Event , while the other three groups did not drop to 50%.
Univariate analysis in all breast cancer subgroups showed that RCB can predict the prognosis of patients very well. With the increase of its value, the risk of recurrence, metastasis or death of patients is significantly increased .
In the multivariate analysis after correction, RCB can also independently and significantly predict patients’ EFS and DRFS.
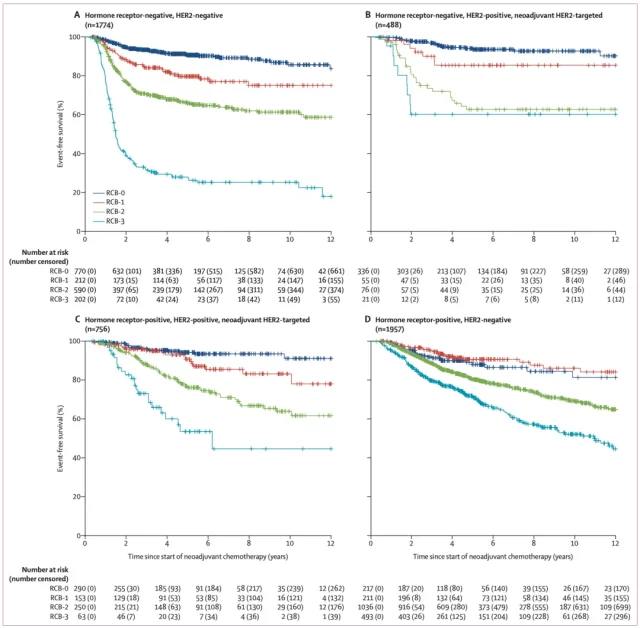
▲ RCB level and EFS curves of different breast cancer subtypes
In addition, RCB has different effects on different subgroups of breast cancer.
The least impact is in patients with hormone receptor-positive and HER2-negative breast cancer. Each increase in RCB score by 1 unit increases the risk of recurrence, metastasis or death of this type of breast cancer52 % (HR: 1.52) .
The greatest impact is in patients with hormone receptor-negative HER2-positive breast cancer. Each increase in the RCB score increases the risk of recurrence, metastasis or death of this type of breast cancer by 109% (HR: 2.09) .
Overall, this study shows that RCB has great potential in predicting the prognosis of neoadjuvant therapy for breast cancer.
However, this study also has some limitations. For example, the neoadjuvant treatment plan and treatment duration in each clinical study are not exactly the same, the follow-up time is different, and the proportion of breast lobular carcinoma included in the study is low.
However, the flaws are not concealed, because this study has achieved a high degree of consistency in different research centers and different breast cancer subgroups, so the research results are highly reliable.
In fact, there are currently three commonly used methods for pathological examination of neoadjuvant treatment of breast cancer, the Miller-Payne system, the RCB system and the American Joint Committee on Cancer (AJCC) ypTNM staging.
It is generally believed that ypTNM staging is the most concise and clear, and can provide information about tumor size, lymph node status, etc., but there is insufficient quantitative information for lesions.
The Miller-Payne system can provide information on pathological changes before and after surgery. The evaluation of drug sensitivity is more in-depth and detailed than the RCB system.
However, the acquisition of tumor tissue before surgery is usually a small part of the tissue, and the comparison result has a greater subjective Factors, there is a lack of large-scale clinical studies to confirm its prognostic value.
The RCB system has good objectivity and maneuverability, but there are some blind areas in RCB. For example, when the tumor invades the skin or chest wall, the RCB cannot assess the prognosis well.
Therefore, the RCB combined with the AJCC staging system may provide a more comprehensive assessment of the neoadjuvant. Efficacy of chemotherapy .
references:
[1] Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005 Feb 2;97(3):188-94. doi: 10.1093/jnci/ dji021. PMID: 15687361.
[2] Cortazar P, Zhang L, et a l. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 Jul 12;384(9938):164-72. doi: 10.1016/ S0140-6736(13)62422-8. Epub 2014 Feb 14. Erratum in: Lancet. 2019 Mar 9;393(10175):986. PMID: 24529560.
[3] Yau C, Osdoit M, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2021 Dec 10:S1470-2045(21)00589 -1. doi: 10.1016/S1470-2045(21)00589-1. Epub ahead of print. PMID: 34902335.
Residual tumor burden after neoadjuvant chemotherapy for breast cancer.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



