Avelumab combined with cetuximab for the treatment of advanced anal squamous cell carcinoma
- Engineered Soybeans with Pig Protein: A Promising Alternative or Pandora’s Dish?
- Severe Fever with Thrombocytopenia Syndrome (SFTS): A Tick-Borne Threat with High Mortality
- Why Isolating Bananas Extends Their Shelf Life?
- This common vitamin benefits the brain and prevents cognitive decline
- New report reveals Nestlé adding sugar to infant formula sold in poor countries
- Did Cloud Seeding Unleash a Deluge in Dubai?
Avelumab combined with cetuximab for the treatment of advanced anal squamous cell carcinoma
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Avelumab combined with cetuximab for the treatment of advanced anal squamous cell carcinoma, hope is emerging.
Squamous cell carcinoma of the anal canal is a rare malignancy with a poor prognosis in advanced stage patients and no standard therapy after failure of first-line therapy.
The Journal for ImmunoTherapy of Cancer published a phase II, non-comparative, randomized study called CARACAS using a “pick-and-win” design of Avelumab alone or in combination with cetuximab in previously treated patients with advanced disease In patients with squamous cell carcinoma of the anal canal, the combined group of results showed promising prospects, providing a potential option for this disease with a huge clinical need.

Patients with advanced anal canal squamous cell carcinoma have poor prognosis and no standard treatment after progression on first-line therapy
Although squamous cell carcinoma of the anal canal (SCAC) is a rare malignancy, accounting for approximately 2% of all gastrointestinal tumors, its annual incidence has steadily increased over the past 15 years.
About 90% of cases are associated with human papillomavirus (HPV) infection, and other risk factors include smoking, HIV, or other causes of immunosuppression.
Based on limited evidence from retrospective case series, treatment of patients with advanced SCAC (aSCAC) has historically been based on palliative chemotherapy with a combination of cisplatin and 5-fluorouracil (5-FU). Recently, carboplatin/paclitaxel became another standard first-line treatment option based on the results of the randomized phase II InterAACT study.
In a single-arm phase II clinical trial, standard or modified-dose DCF (docetaxel, cisplatin, and 5-FU) regimens showed high disease control rates (DCR) in chemotherapy-naive advanced SCAC and durable progression-free survival (PFS), it is another option for first-line treatment in patients with good clinical condition.
The prognosis of patients with aSCAC is poor, and there are no evidence-based treatment options after failure of first-line chemotherapy.
We therefore designed the CARACAS study to evaluate the efficacy and safety of the PD-L1 monoclonal antibody Avelumab alone or in combination with cetuximab in previously treated patients with locally advanced or metastatic SCAC.
The CARACAS study was divided into two groups, but it was not comparative, using a “choose a winner” strategy
The CARACAS study is an open-label, non-comparative, “select-winner”, multicenter, randomized, phase II clinical study involving 17 research centers in Italy.
Main inclusion criteria: ≥18 years old, histologically diagnosed as SCAC, ECOG PS score 0-2, with measurable lesions according to RECIST V.1.124, having received at least first-line treatment for metastatic disease before, liver, kidney, Bone marrow function is good.
Treatment regimen: Enrolled patients were randomized 1:1 to Avelumab alone (arm A) or in combination with cetuximab (arm B). Group A: Avelumab 10mg/kg IV d1, once every 2 weeks; group B, cetuximab 500mg/m 2 IV d1 + Avelumab 10mg/kg IV d1, once every 2 weeks. Both groups were treated until disease progression, patient refusal, or unacceptable toxicity.
Study endpoints: As a formal comparison of treatments between the two study groups was not allowed, the results for each group are described separately.
The primary endpoint was objective response rate (ORR). The one-sided alpha error was set to 0.05, the power of the test was 80%, and at least 4 responses in each group of 27 patients had to be observed to declare the study positive. Secondary endpoints were PFS, overall survival (OS) and safety.
Group B (Avelumab + cetuximab) study endpoint reached: ORR 17%
From September 18, 2018, to July 2, 2019, a total of 60 eligible patients were enrolled, 6 more than planned enrollment, to offset possible withdrawals. There are 30 cases in each group.
Baseline characteristics of patients were largely balanced between the two groups, except for a higher proportion of women in group A (80% in group A and 57% in group B). The median age was 63 years.
There were 12 cases (40%) in each group with distant metastasis; 7 cases (23%) in group A and 10 cases (33%) in group B had received ≥ 2 lines of therapy;
3 cases (10%) in group A and group B 1 patient (3%) had HIV infection;
HPV status was centrally assessed in 56 (93%) patients, 28 in each group, 25 (89%) HPV-positive in group A and 26 (93%) HPV-positive in group B Positive.
Data as of September 21, 2020, with a median follow-up of 17.2 months. Efficacy assessments were performed in 57 patients, as 3 (1 in Group A, 2 in Group B) died of disease prior to the first imaging reassessment.
In the intention-to-treat (ITT) population of 30 patients in each group, 3 patients in group A and 5 patients in group B achieved partial remission (PR), with ORRs of 10% and 17%, respectively, and the combination group achieved the primary endpoint.
A waterfall plot of tumor response is shown in Figure 1. In the ITT population, the disease control rate (DCR) was 50% in group A and 57% in group B, and the median duration of disease control was relatively durable, 4.2 months and 4.6 months, respectively.
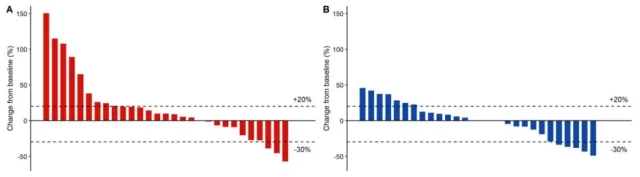
Figure 1. Tumor diameter change from baseline in groups A (A) and B (B)
As of September 21, 2020, the median PFS was 2.0 months in group A and 3.9 months in group B; the median OS was 12.8 months in group A and 7.8 months in group B.
Data as of July 15, 2021, with a median follow-up of 26.7 months, updated data to show: median OS was 13.9 months in arm A and 7.8 months in arm B (Figure 2A, B); median PFS A It was 2.0 months in group B and 3.9 months in group B (Fig. 2C,D).
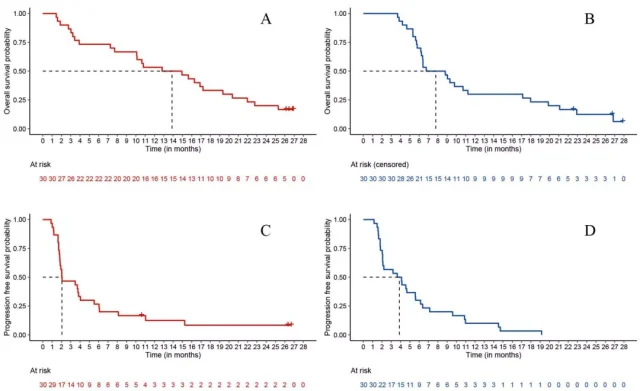
Figure 2. PFS and OS Kaplan-Meier curves. A. OS in group A; B. OS in group B; C. PFS in group A; D. PFS in group B
Safety was acceptable in both groups
The most common adverse event (AE) in arm A was fatigue (17%) and arm B was cutaneous and subcutaneous disease (87%). No grade 3-4 treatment-related AEs were observed in arm A;
the most common grade 3-4 treatment-related AEs in arm B were cutaneous and subcutaneous disease, hypomagnesemia, and aspartate/alanine aminotransferase elevations , the incidence rate is 6%. No toxic fatalities reported.
In conclusion
For SCAC patients, there is currently no standard therapy after failure of standard first-line chemotherapy. And no randomized studies on second-line therapy have been published to date.
Unfortunately, conducting clinical trials in this setting is indeed limited by the rarity of the disease, the short life expectancy, and the fact that clinical conditions are often hampered by the aggressive behavior, complications, and associated comorbidities of HIV infection in this malignancy.
On the basis of fully understanding the clinical needs and combining the mechanism of action of PD-(L)1 and anti-EGFR mAbs, the researchers designed this study to explore the anti-PD-L1 mAb Avelumab alone or in combination with anti-EGFR mAbs Activity of cetuximab.
The study design was a non-comparative randomized phase II clinical trial using an ORR-based “choose a winner” strategy. Results The primary endpoint of the combination arm was achieved with an ORR of 17%.
Moreover, the duration of tumor regression and disease stabilization in the combination group was also more durable, and the PFS of 3.9 months was also encouraging.
It can be estimated from the Kaplan-Meier model that about 30% of patients had no progression for at least 6 months, and 10% had no progression for at least 6 months.
1 year without progress, indicating that this therapeutic strategy still has potential.
OS results should be interpreted with caution because of the small sample size and slight imbalance in baseline characteristics between groups, such as the higher proportion of women in group A, a well-known positive prognostic indicator in SCAC.
Other factors that may have influenced OS were a slight imbalance in disease burden or number of previous lines of treatment at enrollment between the two groups.
In addition, looking at the shape of the OS curves, although the median OS was shorter in group B, overall OS results were similar, as the curves of the two groups crossed at 6 months and were very close after 18 months.
No major differences in post-study treatment were observed, with 11 patients in each group receiving further treatment.
In conclusion, the CARACAS study met its primary endpoint in the cetuximab-Avelumab combination arm, showing promising activity in aSCAC with dual blockade of EGFR and PD-L1 with a manageable safety profile.
References
Lonardi S, Prete AA, Morano F, et al. Randomized phase II trial of Avelumab alone or in combination with cetuximab for patients with previously treated, locally advanced, or metastatic squamous cell anal carcinoma: the CARACAS study. J Immunother Cancer. 2021 Nov ;9(11):e002996. doi: 10.1136/jitc-2021-002996. PMID: 34815354; PMCID: PMC8611452.
Avelumab combined with cetuximab for the treatment of advanced anal squamous cell carcinoma
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



