Journal Clinical Oncology: Immunotherapy saves another type of cancer patient!
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
- Kochi University pioneers outpatient bladder cancer treatment using semiconductor lasers
- ASPEN 2024: Nutritional Therapy Strategies for Cancer and Critically Ill Patients
- Which lung cancer patients can benefit from neoadjuvant immunotherapy?
- Heme Iron Absorption: Why Meat Matters for Women’s Iron Needs
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
Clinical Oncology: Immunotherapy saves another type of cancer patient!
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Journal Clinical Oncology: Immunotherapy saves another type of cancer patient!
Endometrial cancer is the second most common female reproductive system malignancy, and its incidence is second only to cervical cancer.
Platinum-based chemotherapy is the standard first-line treatment for advanced and recurrent endometrial cancer. However, there is still no standard second-line treatment for patients who are not sensitive to platinum drugs, so the overall 5-year survival rate of patients with advanced and recurrent endometrial cancer is only 17% [1,2].
However, the good news for patients with advanced endometrial cancer is finally here! This time, it is the immune checkpoint inhibitor with outstanding achievements that breaks the treatment dilemma!
Recently, Dr. David M. O’Malley and his clinical research team from The Ohio State University James Cancer Center reported in the “Journal of Clinical Oncology” that pembrolizumab was used for microsatellite highly unstable (MSI-H). )/mismatch repair deficient (dMMR) long-term follow-up results of second-line treatment in patients with advanced endometrial cancer [3].
The follow-up results from the clinical phase II KEYNOTE-158 study showed that the median progression-free survival (PFS) of patients after treatment was 13.1 months, the four-year overall survival (OS) rate was as high as 60%, and the objective response rate (ORR) was as high as 60%. ) reached 48%, and 68% of patients who achieved remission had a remission of 3 years or more , and the efficacy of pembrolizumab was excellent.

The success of pembrolizumab is largely due to the fact that 25-31% of patients with endometrial cancer are MSI-H/dMMR positive. Previous clinical studies have found that pembrolizumab is effective against a variety of MSI-H/dMMR-positive advanced solid tumors, especially in endometrial cancer, pembrolizumab can shrink tumors in 70% of advanced patients by 30% % above [4].
With its outstanding efficacy, pembrolizumab, which is used for second-line treatment of advanced or unresectable MSI-H/dMMR-positive tumors for any indication of any cancer type, was also approved by the FDA for accelerated approval as early as 2017. Mainly based on multiple single-arm clinical studies including KEYNOTE-158 in multiple MSI-H/dMMR-positive advanced cancers.
To further explore and validate the efficacy of pembrolizumab in the second-line treatment of recurrent or advanced MSI-H/dMMR-positive endometrial cancer, the researchers evaluated 90 patients with advanced MSI-H/dMMR endometrial cancer included in the KEYNOTE-158 study. Cancer patients were analyzed separately.
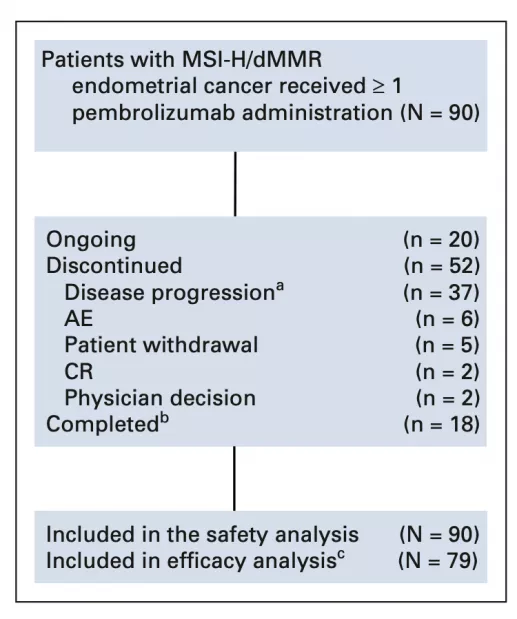
Distribution of trial patients
Of the 90 patients, 48% had received 2 or more prior rounds of treatment, and 68% had received radiation therapy. Patients were followed up for an average of 42.6 months. Pembrolizumab was administered at a dose of 200 mg every three weeks in this trial, and patients received pembrolizumab for an average of 8.3 months, with 20% of patients receiving up to 35 cycles .
Seventy-nine patients who received pembrolizumab for more than 26 weeks were included in the efficacy analysis. Forty-eight percent of patients achieved an objective response (95% CI, 37% to 60%), with 14% (11/79) having a complete response and 34% (27/79) having a partial response. The ORRs were 53% and 44% for patients who had received 1 cycle of therapy and 2 or more cycles, respectively.
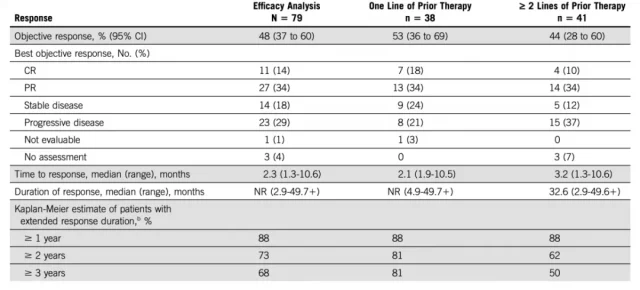
Objective Mitigation Data Aggregation
Surprisingly, of the 38 patients who achieved an objective response, 21 patients remained in response, 8 of whom continued to maintain a complete response with a median duration of response (DoR) not yet reached;88 % of patients had remissions lasting more than a year, 73% lasting more than two years, and 68% lasting more than three years.
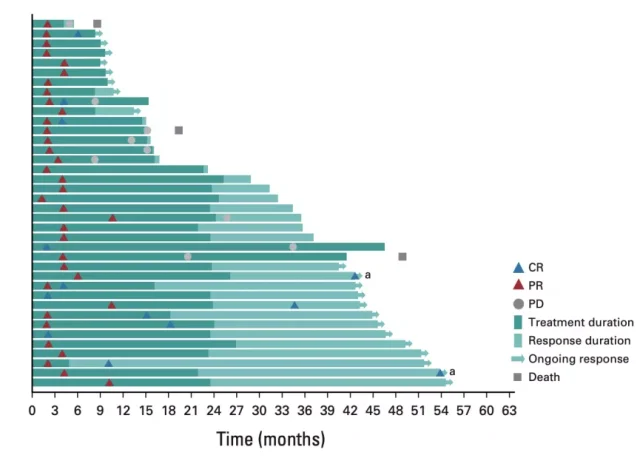
“Swimming” diagram of disease process distribution in 38 patients who achieved objective response
The median PFS of patients included in the analysis was 13.1 months (95% CI, 4.3 – 34.4 months), and the overall 1-, 2-, and 3/4-year progression-free survival rates were 51%, 41%, and 37%, respectively. %; the median overall survival (OS) of the patients has not yet been reached, and the overall 1-year, 2-year, and 3/4-year overall survival rates of the patients were 69%, 64%, and 60%, respectively, showing a good effect of immunotherapy. Long-term survival benefit.
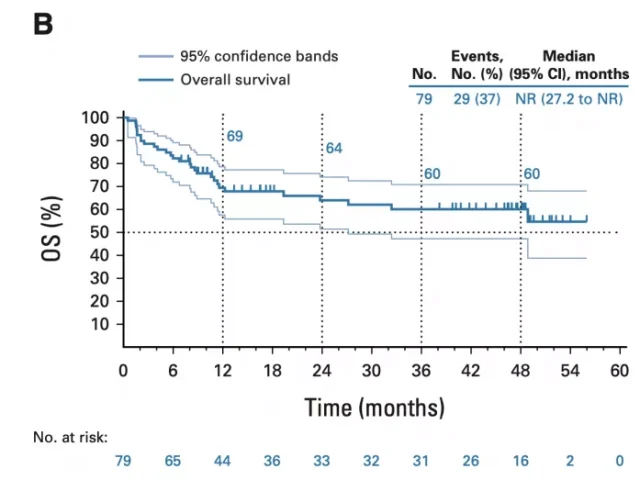
overall survival curve
In terms of safety, no unexpected adverse events were found during long-term follow-up. Of all 90 patients included in the analysis, 76% experienced treatment-related adverse reactions, of which 12% were grade 3-4 adverse reactions; 28% of patients experienced immune-related adverse reactions, of which 7% were 3-4 level event. Only 7% of patients discontinued due to adverse reactions, and none of the adverse events leading to death occurred in the trial.
In conclusion, pembrolizumab can bring long-term deep remission to patients with advanced MSI-H/dMMR endometrial cancer . Nearly half of the patients can achieve objective remission, and the remission duration can be as long as more than three years. New safety signals were found, suggesting that the safety of the treatment is controllable.
In addition to pembrolizumab, there are a variety of PD-1/PD-L1 inhibitors that have demonstrated efficacy against MSI-H/dMMR advanced or recurrent endometrial cancer, such as the PD-1 inhibitor dostarlimab , PD-L1 inhibitors durvalumab, avelumab, etc. [5-8], among which dostarlimab has been approved by the FDA for accelerated approval by virtue of the data of its phase I GARNET trial.
In addition, for non-MSI-H/dMMR advanced endometrial cancer, immune checkpoint inhibitors have also begun to emerge. For example, the combination therapy of pembrolizumab and lenvatinib was officially approved by the FDA last year for non-MSI-H/dMMR advanced or recurrent endometrial cancer that has received previous treatment. Compared with traditional chemotherapy This combination has demonstrated significant efficacy advantages in phase III clinical trials [9].
In the era of tumor immunotherapy in full bloom, the treatment of advanced endometrial cancer is opening a new chapter full of hope!
references:
1. Siegel RL, Miller KD, Fuchs HE, et al: Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021
2. Rousset-Rouviere S, Rochigneux P, Chrétien AS, et al. Endometrial Carcinoma: Immune Microenvironment and Emerging Treatments in Immuno-Oncology. Biomedicines. 2021;9(6):632.
3. O’Malley DM, Bariani GM, Cassier PA, et al. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study [published online ahead of print, 2022 Jan 6]. J Clin Oncol . 2022; JCO2101874.
4. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38(1 ): 1-10.
5. Oaknin A, Tinker AV, Gilbert L, et al: Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: A nonrandomized phase 1 clinical trial. JAMA Oncol 6:1-7, 2020
6. US Food and Drug Administration: FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Endometrial Cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab -gxly-dmmr-endometrial-cancer
7. Antill YC, Kok PS, Robledo K, et al: Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). J Clin Oncol 37, 2019 (suppl ; abstr 5501)
8. Konstantinopoulos PA, Luo W, Liu JF, et al: Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol 37:2786-2794, 2019
9. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med, 2022.
Journal Clinical Oncology: Immunotherapy saves another type of cancer patient!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



