FDA Approved Second mRNA COVID-19 Vaccine: Spikevax
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
FDA Approved Second mRNA COVID-19 Vaccine: Spikevax
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA Approved Second mRNA COVID-19 Vaccine: Spikevax.
Moderna announced today that the U.S. Food and Drug Administration (FDA) has approved the Biologics License Application (BLA) for the company’s mRNA COVID-19 vaccine, Spikevax (mRNA-1273), for the prevention of COVID-19 virus infection in people over 18 years old. The trade name is Spikevax.
This is the second mRNA vaccine officially approved by the FDA, and the first product of Moderna to be officially approved by the FDA.
Boosted by this news, Moderna’s U.S. stock market closed up 6.18% on Monday. As of press time, the stock rose 1.28% after the market to $171.50.

Spikevax, a COVID-19 vaccine that uses mRNA to encode the new coronavirus spike protein, has been granted emergency use authorization (EUA) by the U.S. FDA on December 18, 2020, for the prevention of COVID-19 in individuals over 18 years of age.
This approval is based on the FDA’s evaluation and analysis of follow-up safety and efficacy data from ongoing randomized, blinded, placebo-controlled clinical trials supporting the company’s EUA.
And real-world data after EUA to further test the safety and efficacy of the vaccine.
The latest analysis to determine the effectiveness of Spikevax included 14,287 vaccine recipients and 14,164 placebo recipients, who were over 18 years of age and had no evidence of COVID-19 infection prior to receiving the first dose.
The data used for the analysis were accumulated before the Omicron variants emerged.
These data show that Spikevax is 93.2% effective in preventing symptomatic COVID-19 , including 93.4% for people aged 18-65 and 91.5% for people over 65.
There were 55 cases of COVID-19 in the vaccine group and 744 cases in the placebo group.
The vaccine was also 98.2 percent effective in preventing severe disease.
Major side effects include injection site reactions, joint pain, vomiting, fatigue, headache, muscle pain, chills, injection site swelling, and fever.
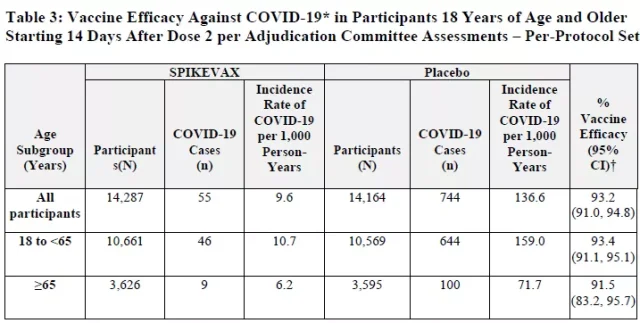
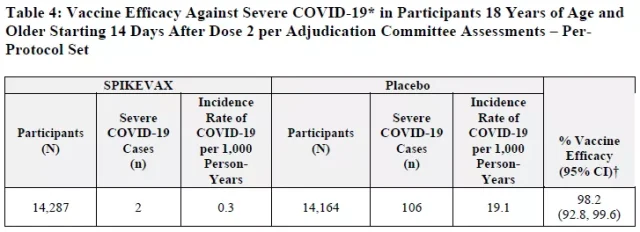

The FDA reviewed several months of additional follow-up data submitted by Moderna to confirm the effectiveness of its vaccine against the new coronavirus.
The FDA also analyzes and pays close attention to cases of very serious side effects after vaccination, including rare heart inflammation.
The disease is more common in young men after the second vaccination, and most cases are mild and resolve quickly.
In addition, the FDA reviewed Moderna’s vaccine manufacturing process and facilities.
” This is an important milestone in Moderna’s corporate history, ” Moderna CEO Stéphane Bancel said in a statement . ” The public can rest assured that this vaccine was approved under the FDA’s rigorous scientific standards,” Dr. Peter Marks, the FDA’s top vaccine regulator, said in a statement . “
Following full approval, Moderna will now be marketed under the brand name Spikevax.
This is the Cambridge, Massachusetts, company’s first FDA-approved product. Currently, Johnson & Johnson has not applied for full approval of the Covid-19 vaccine.”
Moderna’s Covid-19 vaccine has been available for more than a year in the U.S. and global markets under an Emergency Use Authorization (EUA).
Moderna is now fully licensed, which means the manufacturer can advertise its vaccine directly to patients
. It will be sold under the Spikevax brand name. Moderna’s COVID-19 vaccine is also the second licensed COVID-19 virus vaccine in the United States after Pfizer’s (PFE.US)/BioNTech (BNTX.US) COVID-19 vaccine.
FDA Approved Second mRNA COVID-19 Vaccine: Spikevax
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



