Why didn’t NKT cell-based immunotherapy have obvious effects?
- Did Cloud Seeding Unleash a Deluge in Dubai?
- Scientists Identify Gut Bacteria and Metabolites that Lower Diabetes Risk
- OpenAI’s Model Matches Doctors in Assessing Eye Conditions
- UK: A Smoke-Free Generation by Banning Sales to Those Born After 2009
- Deadly Mutation: A New Monkeypox Variant Emerges in the DRC
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
Why didn’t NKT cell-based immunotherapy have obvious effects?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Why didn’t NKT cell-based immunotherapy have obvious effects?
At present, tumor immunotherapy is in the ascendant, and one of the most effective treatment strategies is adoptive cell transfer therapy ( ACT ). Chimeric antigen receptors ( CARs ) and engineered T-cell receptors ( TCRs ) have been the mainstay of adoptive T-cell immunotherapy in recent years.
Natural killer T ( NKT ) cells are a subset of lipid-reactive T cells that enhance antitumor immunity. Immunotherapy based on NKT cells has shown promising therapeutic effects in preclinical, but no obvious effect has been achieved in clinical treatment.
At present, some new treatment strategies are being developed and applied to NKT cell therapy, which is expected to achieve breakthroughs in future clinical treatment.
NKT cells
NKT cells are a subset of lipid- and glycolipid-responsive T lymphocytes that co-express markers associated with NK cells ( NKp46, NK1.1 ). NKT cells play an important role in tumor immune surveillance and anti-tumor immunity. Unlike traditional T cells that recognize peptide antigens by MHC I or II, NKT cells recognize endogenous and exogenous glycolipids presented through the MHC I-like molecule CD1d.
Based on TCR rearrangement and glycolipid reactivity, NKT cells can be divided into two major subpopulations: type I and type II NKT cells. Type I NKT ( also known as iNKT ) cells express a constant rearranged TCRα chain with a restricted TCRβ chain. In mice, Vα14Jα18 ( TRAV11-TRAJ18 ) was paired with Vβ7 ( TRB29 ), Vβ8.2 ( TRB13-2 ) or Vβ2 ( TRBV1 ); in humans, Vα24Jα18 ( TRAV10-TRAJ18 ) was paired with Vβ11 ( TRBV25-1 ).
After recognizing glycolipid antigens, iNKT cells rapidly secreted immunomodulatory cytokines, including IFN-γ, TNF, and IL-4, to affect downstream immune activity. Human iNKT cells can be CD4, CD8 double negative ( CD4-CD8- ), while mouse NKT cells are CD4+ or CD4-CD8-. In addition, iNKT cells can differentiate into NKT1, NKT2, and NKT17 subsets, similar to Th1, Th2, and Th17 T cell subsets.
Type II NKT cells have more diverse Vα rearrangement sequences ( including TRAV7, TRAV9, and TRAV12 ) that help recognize self lipids, such as cerebroside sulfate or lysophosphatidylcholine.
Multiple studies have shown that activation of type II NKT cells by administration of cerebroside sulfate increases tumor growth and enhances metastasis, suggesting a pro-tumor effect, while iNKT cells exhibit potent anti-tumor activity.
Antitumor effects mediated by iNKT cells
Activated iNKT cells can provide antitumor immunity through four mechanisms: direct tumor lysis, recruitment and activation of cytotoxic innate and adaptive immune cells, suppression of suppressor cells in the tumor microenvironment ( TME ), and promotion of tumor-targeted immune memory.
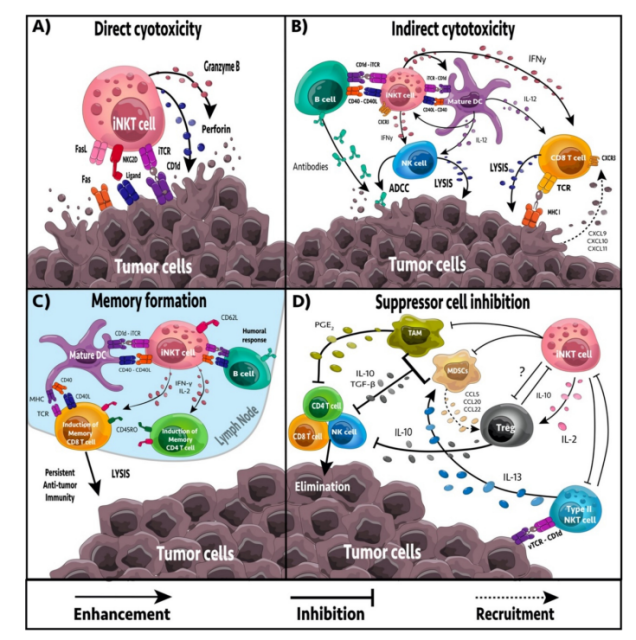
direct or indirect targeting
iNKT cells can target tumor cells directly or indirectly. iNKT cells have the ability to mediate direct cytolytic activity against CD1d-positive tumor cells through the perforin, granzyme B and TNF-related apoptosis-inducing ligand ( TRAIL ) pathways.
In vitro and in vivo studies have shown that iNKT cell-mediated cytotoxicity is associated with increased expression of CD1d on the tumor cell surface, resulting in enhanced tumor lysis and reduced metastasis rate, while downregulation of CD1d is associated with decreased iNKT cell recognition, tumor escape, and increased malignancy.
In the absence of CD1d expression on tumor cells, iNKT cells may be activated by CD1d+ APCs, including dendritic cells ( DCs ), B cells, myeloid-derived suppressor cells ( MDSCs ), and tumor-associated macrophages ( TAMs ). The response of iNKT cells is largely dependent on APCs presenting glycolipids.
Upon activation, iNKT cells secrete numerous pro-inflammatory cytokines, including IL-2, IL-4, IL-17, IFN-γ, and TNF, which affect a wide range of immune cells, including DCs, macrophages, neutrophils, and neutrophils. Granulocytes, NK cells, and T and B cells. Furthermore, activated iNKT cells can actively recruit and induce DC maturation through CD40/CD40L and CD1d/TCR interactions.
Regulation of immunosuppression
TAMs are immunosuppressive immune cells that frequently occur in the TME. TAMs contribute to tumor progression and inhibition of NK, iNKT and T cell responses. In neuroblastoma, iNKT cells can colocalize and lyse TAMs through a CD1d-dependent mechanism. Furthermore, iNKT cells can reprogram M2-polarized TAMs into inflammatory M1 macrophages, reducing TAM-mediated immunosuppression.
MDSC induces NK and T cell anergy, promoting immunosuppression and tumor growth. Furthermore, they can reshape the TME, increasing the transfer rate. In an influenza A infection model, iNKT cells could inhibit arginase-1 and NOS2-mediated inhibitory activity of MDSCs in a CD1d- and CD40-dependent manner. In addition, NKT cells can stimulate the conversion of α-GalCer-loaded MDSCs into mature APCs capable of inducing NK and T cell immune responses.
In addition, type II NKT cells appear more frequently in the TME, have immunosuppressive effects, and increase tumor progression and metastasis. Activation of iNKT cells reduces the number of type II NKT cells.
The formation of tumor immune memory
The generation of immune memory is critical for durable antitumor immune responses and prevention of tumor recurrence. Activation of tumor antigens or cross-reactive exogenous antigens induces naive T cells to expand and differentiate to acquire appropriate effector functions to target cancer cells.
CD8α+ dendritic cells excel in MHC I cross-presentation, and iNKT cells have been shown to directly drive CD8α+ dendritic cells for cross-priming, even in the absence of CD4+ T cells. In multiple tumor models, NKT cell activation has been shown to enhance CD8+ T cell-mediated antitumor immunity.
Although most iNKT cells lack CD62L, a recent study showed that CD62L+ iNKT cells increased and persisted longer than CD62L neg iNKT cells after glycolipid stimulation. CD62L neg iNKT cells acquire an exhausted phenotype ( PD-1, TIM-3, decreased cytokine production ) and rapidly undergo apoptosis, whereas CD62L+ iNKT cells continue to proliferate and produce large amounts of cytokines.
NKT-based cellular immunotherapy
Due to the important role of iNKT cells in immune surveillance and antitumor immunity, iNKT cell-based immunotherapy has become an important research area, and various strategies have been used to target cancer.
Give free α-GalCer
In preclinical trials, free [alpha]-GalCer may be administered prophylactically, concurrently, or after tumor inoculation. After injection of free α-GalCer, iNKT cells rapidly proliferated and produced TNF, IL-2, IL-4, and IL-13, followed by IFN-γ responses, with multiple injections biased toward Th2 responses.
Although administration of free α-GalCer after tumor inoculation had some antitumor effect in multiple mouse models, the antitumor response was limited. Therefore, free α-GalCer is often used in combination with cytokines or other immunotherapies to enhance the therapeutic effect.
In phase I clinical trials, administration of α-GalCer was well tolerated with no dose-limiting toxicities; however, it failed to produce a clinical response, with only 7 SD of 24 patients. Low efficacy may be attributed to low iNKT cell numbers at baseline, as only patients with high iNKT cell numbers exhibited NK cytotoxicity and stable disease.
Adoptive transfer of α-GalCer-presenting DCs
α-GalCer-loaded DCs have been used to overcome the low immunogenicity and anergy induction of free α-GalCer. Loading α-GalCer onto DCs resulted in stronger activation of iNKT cells and less induction of anergy due to co-stimulatory signaling by CD40 and IL-12.
In multiple tumor models, α-GalCer-loaded DCs reduced tumor growth and metastasis, resulting in prolonged survival compared with free glycolipid administration. DCs loaded with α-GalCer increased iNKT proliferation, activation and IFN-γ production. Furthermore, enhanced activation of iNKT cells resulted in increased activation of NK and CD8+ T cells and increased IFN-γ production.
In clinical trials in myeloma and head and neck cancer, mature α-GalCer-loaded APCs were well tolerated with no serious adverse events. Treatment increased IFN-γ production and expansion of iNKT cells, resulting in stable disease and prolonged median survival in many patients.
Treatment with α-GalCer-loaded APCs was well tolerated in phase I and I/II clinical trials in non-small cell lung cancer, resulting in only grade I and II adverse events.
Patients who responded well to treatment exhibited increased IFN-γ production and iNKT cell expansion.
The median survival time was 31.9 months, while the median survival time for all patients was 18.6 months.
Adoptive transfer of activated iNKT cells
Cancer patients often have low numbers and/or reduced function of iNKT cells, limiting the efficacy of α-GalCer therapy. To overcome this limitation, iNKT cells have been isolated from patient-derived PBMCs and adoptively transferred back to patients after in vitro expansion.
In preclinical models, adoptive transfer of activated iNKT cells increases iNKT cell cytotoxicity, tumor regression, and overall survival in a metastatic model of melanoma.
In contrast, in the metastatic 4T1 breast cancer model, adoptive NKT cells alone or in combination with free α-GalCer therapy or α-GalCer-loaded DC did not improve prognosis.
No serious adverse events have been reported in clinical trials using adoptive transfer of iNKT cells for the treatment of non-small lung cancer and advanced melanoma.
Patients had increased numbers of circulating iNKT cells and IFN-γ production, but few patients had decreased tumor progression, suggesting that the treatment was ineffective.
Combining the adoptive transfer of expanded iNKT cells with α-GalCer-loaded DCs increases the efficiency of adoptive NKT cell transfer. Combination therapy resulted in partial responses in some patients compared with iNKT adoptive transfer alone.
As cancer vaccine adjuvant
The role of iNKT cells in recognizing conserved lipid antigens presented through CD1d and their ability to coordinate antitumor immune responses through cytokine signaling make them attractive targets for cancer vaccine development.
Ligands of iNKT cells, such as α-GalCer, can be used together with tumor antigens to act as adjuvants to increase adaptive immune responses against tumors.
In preclinical animal models, α-GalCer in combination with tumor antigens improved overall survival in mice. Intranasal vaccination with α-GalCer and OVA induced humoral and cellular immune responses, resulting in increased cytokine secretion, CTL responses, and IgG production. Currently, there are no registered clinical trials using iNKT cell ligands in cancer vaccines.
CD1d antibody fusion protein
CD1d-positive tumors are more susceptible to iNKT cell-mediated lysis, whereas CD1d downregulation is a common evasion strategy to avoid detection. Therefore, in order to increase the targeting of iNKT cells to tumors, CD1d antibody fusion proteins that direct iNKT cells to tumors were investigated.
Currently, CD1d antibody fusion proteins targeting HER2, CEA and CD19 have been developed. In a HER2-expressing B16 melanoma model, treatment with a HER2-targeted CD1d antibody fusion protein increased iNKT cell inflammatory cytokine production and targeted lysis of tumor cells, reducing metastasis formation.
Furthermore, iNKT cells enhanced the activation of DCs, NK cells, and CD8 T cells, increasing overall immune recruitment and targeting to tumors. Importantly, the CD1d antibody fusion protein has target specificity and shows a good safety profile.
CAR-NKT cells
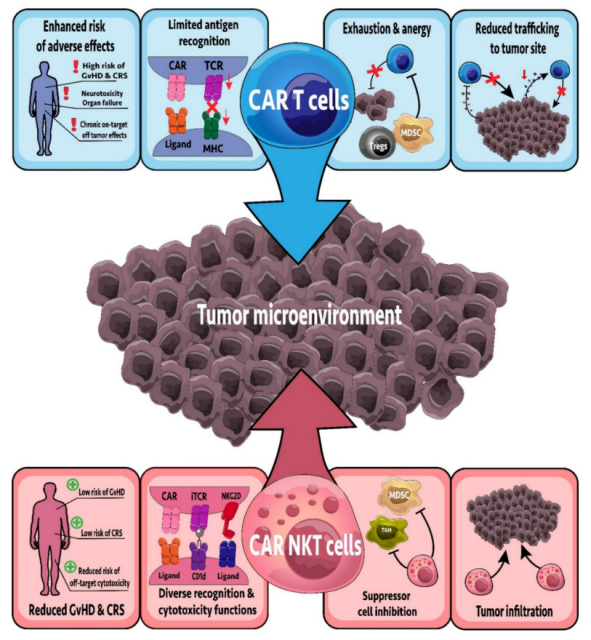
CAR-T cell therapy has achieved remarkable clinical success in targeting B-cell malignancies. iNKT cells have been engineered to express CARs targeting glycolipid and protein antigens.
Furthermore, in addition to CAR, CAR-NKT cells co-expressed a constant TCR, maintaining their responsiveness to glycolipid antigens.
In contrast, the endogenous TCR in CAR-T cells is polyclonal, and most CAR-T cells can only respond through their CAR.
CAR-NKT cells targeting CSPG4, GD2, and CD19 have been developed, and CAR-NKT cells targeting GD2 and CD19 are in clinical trials. A Phase I clinical trial ( NCT03294954 ) has begun to test the efficacy and safety of anti-GD2 CAR-NKT cells in refractory neuroblastoma.
Preliminary results showed that the treatment of 10 enrolled patients was safe, including 1 complete response, 1 partial response, and 3 patients with stable disease.
Currently, CD19 CAR-NKT cells are undergoing a Phase I clinical trial ( NCT03774654 ) testing safety and efficacy in relapsed and refractory B-cell malignancies.
Of the two patients in the phase I trial, one showed a complete response and the other a partial response, and neither patient had any signs of CRS or GvHD.
Importantly, the CAR-NKT cells used in this study were allogeneic, demonstrating the potential for “universal” therapy.
NKT cell-based combination therapy
One way to improve existing treatments is to combine them with other treatments that produce a synergistic response. Combination therapy may also improve the clinical efficacy of iNKT cell immunotherapy. In preclinical trials, iNKT cell immunotherapy has been tested in combination with chemotherapy, oncolytic viruses and other immunotherapies.
Combined chemotherapy
To date, lenalidomide is the only chemotherapy drug used in combination with iNKT cell immunotherapy in clinical trials. In a phase I clinical trial, patients with asymptomatic myeloma received α-GalCer-loaded DC in combination with lenalidomide. Treatment was well tolerated, with only one patient experiencing grade 3 adverse events.
Treatment resulted in increased activation of iNKT cells, NK cells, monocytes, and eosinophils, showing strong innate immune activation. Furthermore, the expression of NKG2D was significantly increased on NK cells, indicating an increased cytotoxic potential of NK cells.
Overall, treatment resulted in lower tumor-associated immunoglobulins in all but one patient, suggesting that treatment reduced tumor burden. The success of the combination therapy underscores the need for further clinical trials.
In combination with oncolytic virus
To date, no clinical trials have combined oncolytic viruses with iNKT cell immunotherapy. However, clinical trials suggest that oncolytic viruses can improve the effects of other immunotherapies. The successful preclinical results of combining oncolytic virus therapy with iNKT cell activation therapy suggest the need to further examine this combination therapy in clinical trials.
Combination immunotherapy
An interesting option for combination therapy is the combination of iNKT cell therapy and immune checkpoint inhibitors. After activation of iNKT cells by α-GalCer, a large amount of IFN-γ is released, followed by increased PD-1 expression, resulting in anergy and inhibition of antitumor function.
Therefore, the combination of iNKT cell immunotherapy and checkpoint inhibitors that block the PD-1/PD-L1 axis may improve the therapeutic effect. Indeed, in preclinical models, α-GalCer combined with anti-PD-1 or anti-PD-L1 prevented iNKT cell anergy and increased the antitumor activity of iNKT cells.
Furthermore, iNKT cell immunotherapy can overcome CD8+ T cell anergy in PD-1-resistant tumors. A clinical trial is testing a combination of PD-1/PDL-1 blockade and iNKT cell immunotherapy ( NCT03897543 ).
Challenges and responses to NKT cell therapy
The biggest challenge for iNKT cell immunotherapy is the low infiltration and reduced function of iNKT cells in cancer patients. Combined with the downregulation of CD1d in tumor cells, therapeutic efficacy may be limited.
Furthermore, iNKT cells induced an anergy phenotype after treatment with free α-GalCer, reducing the efficacy of multi-dose treatment.
Another challenge is obtaining large numbers of autologous DC and iNKT cells from immunosuppressed cancer patients, thereby limiting the number of cells that can be expanded and adoptively transferred.
In addition, adoptive transfer of cultured and differentiated cells takes weeks, leaving some patients unable to receive treatment or dying of the disease. To address these challenges, many studies have improved iNKT cell immunotherapy.
Improve glycolipid delivery
Due to the inefficiency of free α-GalCer and the difficulty in obtaining large numbers of autologous DCs, several studies have examined the possibility of α-GalCer delivery via carriers such as nanoparticles, artificial antigen-presenting cells, exosomes, and liposomes .
Compared with free α-GalCer, delivery of carrier-bound α-GalCer increased iNKT cell expansion and cytokine release, thereby reducing tumor burden. This is mainly due to increased uptake and expression by DC cells, as well as increased downstream NK and CD8+ T cell responses.
Choose Glycolipid Alternatives
Nearly all clinical trials of iNKT cell immunotherapy have used α-GalCer ( KRN7000 ) to stimulate iNKT cells; however, an increasing number of modified glycolipids may offer higher therapeutic benefits. For example, α-C-GalCer replaces the O-glycosidic bonds found in α-GalCer with C-glycosidic bonds, thereby increasing the production of IFN-γ and IL-12.
7DW8-5 is a candidate glycolipid with a short acyl chain terminated by a fluorinated benzene ring. Compared with α-GalCer, 7DW8-5 has a stronger affinity for CD1d and TCR of iNKT cells, and stimulation of iNKT cells with 7DW8-5 increases Th1 and CD8+ T cell responses in various vaccine models.
Similarly, a modified glycolipid called ABX196 has yielded promising results as a vaccine adjuvant. ABX196 alone or in combination with anti-PD-1 increased tumor regression and overall survival in melanoma, colon and bladder cancer models. ABX196 in combination with a PD-1 inhibitor is currently undergoing a Phase 1 clinical trial ( NCT03897543 ).
Induced pluripotent stem cell-derived iNKT cells
Cancer patients often have reduced iNKT cell numbers and function, limiting the efficacy of iNKT cell immunotherapy. Furthermore, it is difficult to obtain sufficient PBMCs from immunosuppressed cancer patients to expand and deliver iNKT cells in vitro. One coping strategy is to use induced pluripotent stem cells ( IPSCs ) from patient tissue. iPSC-induced iNKT cells can secrete large amounts of IFN-γ. Furthermore, in vivo experiments showed that iPSC-derived iNKT cells maintained their antitumor function when transferred into mice.
Targeting type II NKT cells
iNKT cells and type II NKT cells play opposite roles in tumor immune regulation and cross-regulate each other. Therefore, targeting type II NKT cells may be a therapeutic approach to alleviate tumor-mediated immunosuppression of iNKT cells and enhance antitumor immunity. A recently discovered subtype C24:2 of the cerebroside sulfate antigen of type II NKT cells was shown to significantly reduce the occurrence of lung metastases.
Summary
iNKT cells play an important role in immune surveillance and antitumor immunity. NKT cell-based immunotherapy has many advantages.
Compared with CAR-T cell therapy, NKT cell therapy has a longer duration of action and is more capable of inhibiting cancer recurrence and metastasis. It may become an important treatment for solid tumors and other cancers.
Although, there is still no breakthrough in clinical research, some new therapeutic strategies, including carrier-based α-GalCer, alternative glycolipids, and combination therapy, are showing improved preclinical outcomes.
With the continuous breakthrough of immune cell therapy for solid tumors, the NKT cell immunotherapy industry will develop rapidly, and it is believed that NKT cell-based immunotherapy will shine brightly in the future.
references:
1.The Current Landscape of NKT CellImmunotherapy and the Hills Ahead. Cancers (Basel). 2021 Oct; 13(20): 5174.
Why didn’t NKT cell-based immunotherapy have obvious effects?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.