Breast cancer patients with this characteristic may be able to step down treatment to avoid overtreatment and side effects
- Engineered Soybeans with Pig Protein: A Promising Alternative or Pandora’s Dish?
- Severe Fever with Thrombocytopenia Syndrome (SFTS): A Tick-Borne Threat with High Mortality
- Why Isolating Bananas Extends Their Shelf Life?
- This common vitamin benefits the brain and prevents cognitive decline
- New report reveals Nestlé adding sugar to infant formula sold in poor countries
- Did Cloud Seeding Unleash a Deluge in Dubai?
Breast cancer patients with this characteristic may be able to step down treatment to avoid overtreatment and side effects
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Journal of Clinical Oncology: Breast cancer patients with this characteristic may be able to step down treatment to avoid overtreatment and side effects
In recent years, with the continuous improvement of cancer screening awareness and the simple and easy operation of breast cancer screening methods, the screening rate and early incidence of breast cancer have continued to increase.
However, some of the tumors found at screening are indolent tumors, which do not have the ability to progress and metastasize, and do not produce obvious clinical symptoms . These indolent tumors, if unrecognized, lead to overtreatment [1, 2]. For clinicians, there is a clear need for a tool to identify these types of tumors.
The 70-gene detection system (MammaPrint) is a multi-gene detection system for breast cancer developed by the Netherlands Cancer Institute, which is used to assess the risk of distant metastasis in breast cancer patients.
Leonie JMJ Delahaye et al established a new cutoff value in the low-risk category detected by 70 genes to identify patients with ultra-low risk of distant recurrence and found that such ultra-low-risk patients account for 10% of all breast cancers-15 % [3].
Studies have shown that ultra-low-risk breast cancer patients, even in the absence of almost no systemic treatment, have a breast cancer-specific survival rate (BCSS) of 100% at 15 years of follow-up, which indicates that this segment is ultra-low.
Risk characteristics Tumors are extremely indolent [3]. The favorable long-term survival of patients with ultra-low-risk tumors was further demonstrated by Esserman et al in their analysis of patients in the Swedish STO-3 trial [4]. In both studies, however, the numbers of ultra-low-risk patients were small.
Recently, the team of Dr. Laura J. van-t Veer of the University of California published important research results in the Journal of Clinical Oncology (JCO), in the largest prospective MINDACT trial to date, exploratory analysis of ultra-low risk 70 Survival outcomes of genetically characterized patients.
In this study, patients with an ultra-low risk 70-gene signature showed a good prognosis, with an 8-year breast cancer-specific survival (BCSS) rate higher than 99%, few patients developed distant metastasis, and no distant metastasis interval (DMFI). ) reached 8 years in up to 97% of patients [5].
The researchers therefore believe that these patients with the ultra-low-risk 70-gene signature may be candidates for further de-escalation . Through detection, these patients can avoid overtreatment and the resulting side effects, and improve the quality of life of patients without affecting the survival rate.

▲ Screenshot of the homepage of the paper
Next, let’s take a look at how this research is carried out.
The MINDACT trial is a randomized, multicenter, prospective, phase III clinical trial . This study mainly exploratory analysis of the clinical characteristics and prognosis of 1000 ultra-low risk subgroup breast cancer patients in this trial.
From 2007 to 2011, the researchers recruited a total of 6693 patients with early breast cancer to participate in this clinical study, the Adjuvant Online v8.0 clinicopathological system was used to determine clinical risk, and the 70-gene detection system was used to determine genetic risk.
Among 6693 patients, they were divided into high-risk group (2398 cases), low-risk group (3295 cases) and ultra-low risk group (1000 cases) according to the 70-gene test results. Grading.
Of the 1000 ultra-low-risk patients, 67% were >50 years old, 80% were node-negative, 81% had tumors <2 cm, 96% were grade 1 or 2, and 99% were ER positive , 97% were HR+ and HER2-; 84% of patients received systemic therapy (69% endocrine therapy, 14% endocrine therapy plus chemotherapy, 1% other therapy) , and 16% did not receive systemic therapy (AST). In the ultra-low-risk group, 741 (74%) patients had low clinical risk and 259 (26%) patients had high clinical risk .
Next, let’s take a look at the exploratory analysis data of this clinical trial.
At a median follow-up of 8.7 years, grouped according to 70-gene signatures, the 8-year DMFI incidence was 97.0% in the ultra-low-risk group, 94.5% in the low-risk group, and 89.2% in the high-risk group; ultra-low The 8-year incidence of BCSS was 99.6% in the risk group, 98.2% in the low-risk group, and 93.7% in the high-risk group .
Compared with patients in the low-risk group, patients in the ultra-low-risk group had a 35% lower risk of developing distant metastasis or breast cancer-related death .
In a multivariate Cox model adjusted for clinicopathological characteristics and treatment, patients in the high-risk group had a 117% increased risk of death compared with those in the low-risk group.
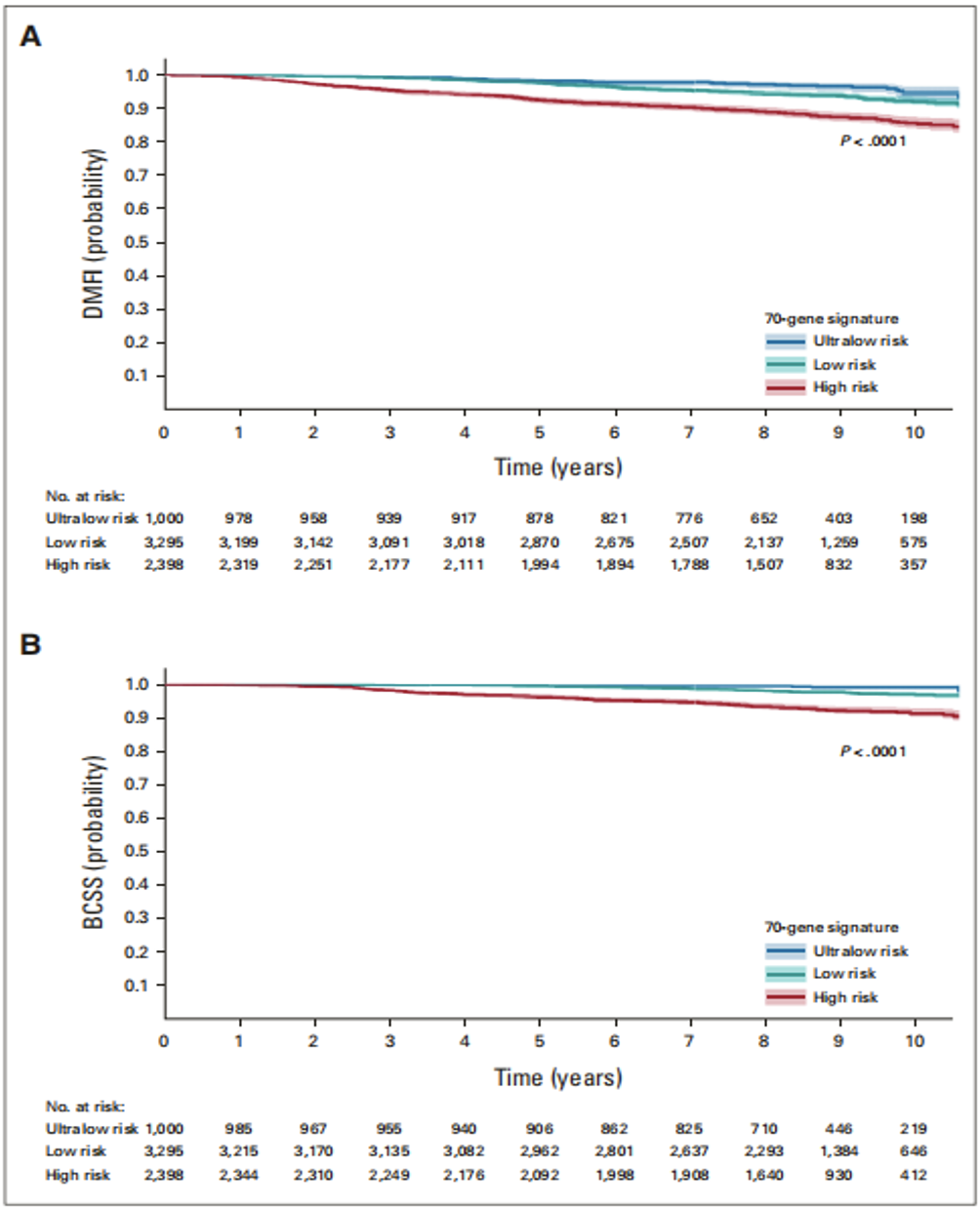
▲ Kaplan-Meier analysis results of DMFI (A) and BCSS (B) of all patients grouped according to 70-gene characteristics
BCSS: breast cancer-specific survival; DMFI: distant metastasis-free interval
The researchers further analyzed the patients in the clinical low-risk group and stratified them according to the 70-gene feature results.
The analysis results showed that the 8-year DMFI incidence rate of the ultra-low-risk group was 97.6%, the low-risk group was 96.3%, and the high-risk group was 96.3%. Group patients was 93.5% .
Genomic hazard ratios in the clinical low-risk group were similar to the overall population, and patients in the ultra-low-risk group had a 31% lower risk of developing distant metastasis or breast cancer-related death compared with patients in the low-risk group , adjusted for clinicopathologic features and treatment.
In the multivariate Cox model of , patients in the high-risk group had a 158% increased risk of death compared with those in the low-risk group.
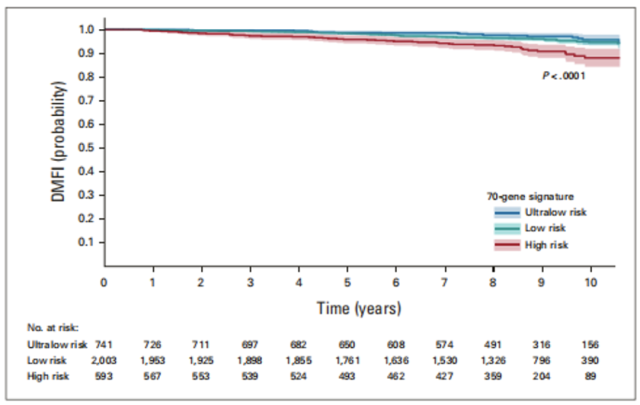
▲ The results of Kaplan-Meier analysis of DMFI for patients in the clinical low-risk group, stratified according to 70-gene characteristics
Subsequently, the researchers conducted a survival analysis of DMFI and BCSS after clinical risk stratification of the 70-gene test ultra-low risk group.
The results showed that the 8-year incidence of DMFI in patients with low clinical risk was 97.6% and that in patients with high clinical risk was 95.0%; at 8 years, the incidence of BCSS in patients with low clinical risk was 99.7%, and the incidence of BCSS in patients with high clinical risk was 99.7%. 99.2%.
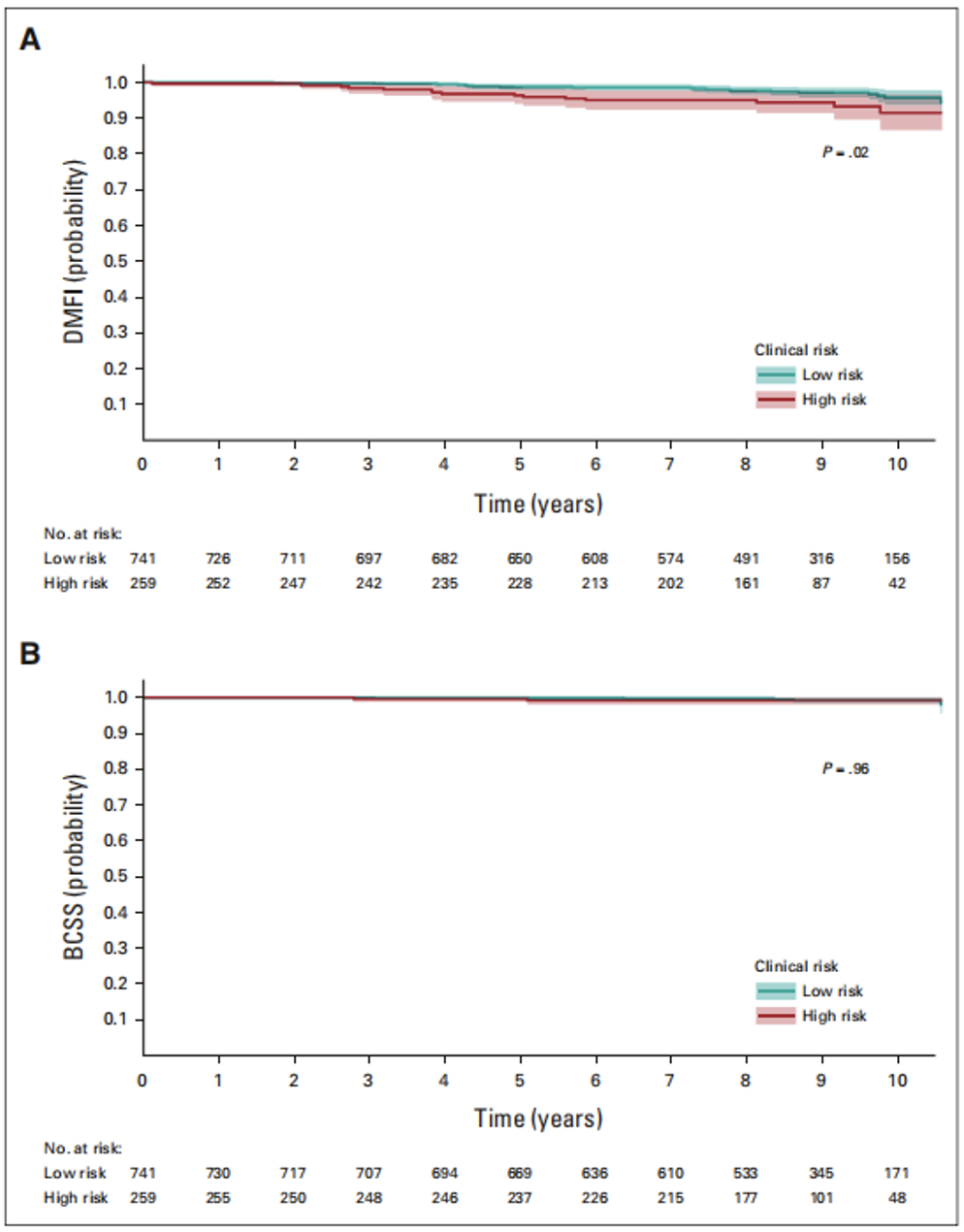
▲ Kaplan-Meier analysis of DMFI (A) and BCSS (B) in ultra-low risk patients by clinical risk
Finally, we exploratory analysis of the effect of receiving systemic therapy (AST) on survival outcomes in a 70-gene ultra-low risk population.
Results of the analysis showed that ultra-low-risk patients who received no AST (97% lower clinical risk) or endocrine therapy (ET) only (83% lower clinical risk) had similarly favorable outcomes, with 8-year DMFI rates respectively. were 97.8% and 97.4% ; patients receiving chemotherapy (92% high clinical risk) had a slightly lower prognosis, with an 8-year DMFI rate of 94.9%.
In the univariate Cox model, in the ultra-low-risk group, only lymph node positivity (regardless of tumor size, grade, age) was significantly associated with the risk of distant metastasis or breast cancer-related death, and node-positive patients had an increased risk of death compared with node-negative patients up 112%.
None of the variables were significantly associated with distant metastasis or breast cancer-related death in multivariate Cox models adjusted for clinicopathological characteristics and treatment.
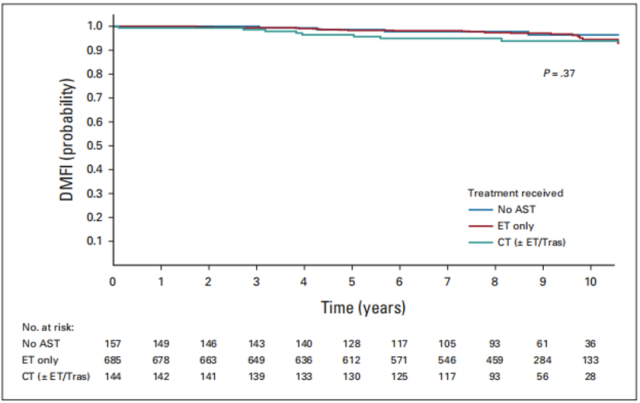
▲ Kaplan-Meier analysis of adjuvant therapy for DMFI in ultra-low risk group patients
AST: adjuvant systemic therapy; ET: endocrine therapy; CT: chemotherapy
These results demonstrate that the 70-gene signature assay can identify ultra-low-risk patients with a favorable prognosis with very low rates of distant recurrence and breast cancer-related mortality, independent of clinical risk ; although most patients did receive AST, The performance of DMFI in the group not receiving AST treatment was consistent with that in the group receiving AST treatment.
Genetic signature testing is now included in international treatment guidelines, and multiple studies have confirmed that genetic signature testing can identify these patient populations who can benefit from it and ultimately prevent overtreatment.
However, current international treatment guidelines still recommend adjuvant endocrine therapy for all HR+ breast cancer patients. To the best of our knowledge, there are no clinical studies evaluating the impact of de-escalation of endocrine therapy on survival in low-risk breast cancer patients with good long-term prognosis at baseline.
Overall, in this largest prospective clinical trial in history, the researchers exploratively analyzed the survival prognosis of the 70-gene ultra-low risk population, providing a new means for further tailoring adjuvant therapy for patients with early breast cancer and ideas .
At the same time, we also hope to see large-scale clinical trials evaluating the impact of endocrine de-escalation on survival outcomes in low-risk breast cancer patients.
References:
1.Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med. 2016;375(15):1438-1447. doi:10.1056/NEJMoa1600249
2.Autier P, Boniol M, Koechlin A, Pizot C, Boniol M. Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ. 2017;359:j5224. Published 2017 Dec 5. doi:10.1136/bmj.j5224
3.Delahaye LJMJ, Drukker CA, Dreezen C, et al. A breast cancer gene signature for indolent disease. Breast Cancer Res Treat. 2017;164(2):461-466. doi:10.1007/s10549-017-4262-0
4.Esserman LJ, Yau C, Thompson CK, et al. Use of Molecular Tools to Identify Patients With Indolent Breast Cancers With Ultralow Risk Over 2 Decades [published correction appears in JAMA Oncol. 2017 Nov 1;3(11):1589]. JAMA Oncol. 2017;3(11):1503-1510. doi:10.1001/jamaoncol.2017.1261
5.Lopes Cardozo JMN, Drukker CA, Rutgers EJT, et al. Outcome of Patients With an Ultralow-Risk 70-Gene Signature in the MINDACT Trial [published online ahead of print, 2022 Jan 21]. J Clin Oncol. 2022;JCO2102019. doi:10.1200/JCO.21.02019
Breast cancer patients with this characteristic may be able to step down treatment to avoid overtreatment and side effects
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



