Biogen acquires full control of Alzheimer’s disease drug Aduhelm!
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
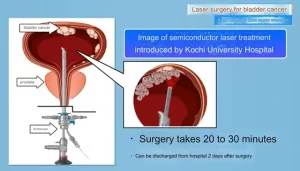
- Kochi University pioneers outpatient bladder cancer treatment using semiconductor lasers
- ASPEN 2024: Nutritional Therapy Strategies for Cancer and Critically Ill Patients
- Which lung cancer patients can benefit from neoadjuvant immunotherapy?
- Heme Iron Absorption: Why Meat Matters for Women’s Iron Needs
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
Biogen acquires full control of Alzheimer’s disease drug Aduhelm!
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Biogen acquires full control of Alzheimer’s disease drug Aduhelm!
Recently, Biogen and Eisai jointly announced that the two parties have revised the existing cooperation agreement on the Alzheimer’s disease (AD) drug aducanumab. The drug is an anti-beta amyloid antibody that will be approved for marketing in the United States in June 2021 under the trade name Aduhelm.

According to an announcement issued by both parties, the amendment to the agreement will take effect on January 1, 2023, and Eisai will receive tiered royalties based on Aduhelm’s net sales, rather than sharing global profits and losses. The royalty rate will start at 2% and will be 8% when sales exceed $1 billion in the year.
From now on, Biogen’s existing final decision-making authority over Aduhelm has been transformed into the sole decision-making authority and commercialization authority on a global scale.
Overall, the two companies’ economic arrangements for 2022 will remain substantially unchanged, with Eisai’s share of expenses for the period January 1-December 31, 2022 capped at those related to the development, commercialization and manufacturing of Aduhelm the agreed amount.
Once the tiered royalty model begins next year, Eisai will not participate in Aduhelm’s economic activities other than these royalties.
In addition, the two companies will continue to jointly develop and commercialize another AD monoclonal antibody, lecanemab, an investigational anti-amyloid beta (Aβ) fibrillar antibody that received U.S. approval in June 2021. FDA Breakthrough Drug Designation.
Eisai will continue to lead the development and regulatory submission of lecanemab globally, and the two companies will co-commercialize and co-promote the product, with Eisai having the final say. The two sides share the economic situation equally, Eisai will record all sales of lecanemab, and Biogen will reflect its 50% profit and loss share.
In addition, the supply agreement related to lecanemab has been extended from 5 years to 10 years.
Biogen will manufacture the lecanemab API at its Solothurn facility in Switzerland, with the goal of providing a reliable commercial supply worldwide.
In response to this revision of the agreement, Biogen stated that this revised cooperation agreement will be able to improve operational efficiency and flexibility in response to market developments, including the Centers for Medicare and Medicaid Services (CMS) finalization of Aduhelm health insurance coverage. Decide.
Reference:
1、Biogen takes full control of troubled Aduhelm in revamped Eisai deal
2、Biogen and Eisai amend collaboration agreements on Alzheimer’s disease treatments
Biogen acquires full control of Alzheimer’s disease drug Aduhelm!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.