Will PCSK9 inhibitors become the main lipid-lowering drugs in the future?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
Will PCSK9 inhibitors become the main lipid-lowering drugs in the future?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Will PCSK9 inhibitors become the main lipid-lowering drugs in the future?
The global morbidity and mortality of cardiovascular disease (CVD) remain high all year round, accounting for about one-third of global deaths, and under the influence of metabolic risk factors such as obesity, dyslipidemia, type II diabetes, and hypertension , has become a huge challenge and a heavy burden to public health and family life.
Among them, atherosclerotic cardiovascular disease (ASCVD) has received more attention. Ischemic heart disease and stroke, which are clinical manifestations of ASCVD, are the main causes of death, accounting for 84.9% of cardiovascular disease deaths.
The annual number of deaths in CVD patients is expected to increase to nearly 24 million by 2030, and the myocardial infarction or stroke experienced by ASCVD patients is inextricably linked to high plasma cholesterol levels.
The correlation between high cholesterol and increased risk of cardiovascular events is mainly related to low-density lipoprotein-cholesterol (LDL-C), LDL is converted from very low-density lipoprotein particles (VLDL), and LDL carries cholesterol to form LDL-C and transport it to Peripheral tissues, also including the vessel wall beneath the dysfunctional endothelium, are oxidatively stressed in the space below the vessel wall, promoting the oxidation of LDL to ox-LDL, which activates the inflammasome of macrophages (NLRP3) to enhance vascular inflammation and ASCVD.
Statins (such as Lipitor) are clinical first-line therapy designed to block cholesterol synthesis as inhibition of the rate-limiting enzyme for cholesterol synthesis, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase Statins potently lower total cholesterol (TC) and low-density lipoprotein (LDL).
However, this therapy is not appropriate for adults with primary hypercholesterolemia (heterozygous family HeFH, non-heterozygous family HoFH) and statin-intolerant patients who often require additional lipid lowering Medications to achieve LDL-C goals.
Discovery of PCSK9
Thus, in 2003, when a French team led by Catherine Boileau and a Canadian team led by Nabil G Seidah worked together to reveal that the human proprotein convertase subtilisin 9 (PCSK9) encodes the autosomal dominant familial hypercholesterolemia (FH3) A third gene, and after regulating LDL-C independently of the LDLR (low density lipoprotein receptor) and apolipoprotein B (APOB) genes, PCSK9 has three binding domains:
- Prodomain (aa 31-152)
- catalytic domain (aa 153-421);
- Hinge-CHRD domain (aa 422-692).
PCSK9 and LDLR mainly interact through their catalytic domains and EGF-A domains, respectively, and the CHRD of PCSK9 is required to trigger the degradation of LDLR, which has triggered a wave of research and development of PCSK9 inhibitors by domestic and foreign pharmaceutical companies: Monogenic Pharmaceuticals, AFFiRiS, AstraZeneca, Betagenon, Merck, Pfizer, as well as domestic companies Cinda, Ke Feiping, Hengrui, Junshi, Xinlitai, Ruibo Bio, etc. are all in this field.
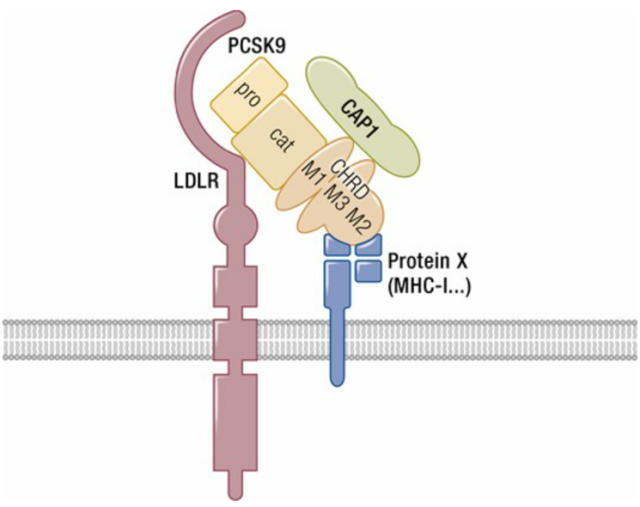
Figure 1. Schematic diagram of PCSK9 and its potential binding sites
Existing PCSK9 inhibitor drugs
PCSK9 inhibitors reduce circulating LDL-C levels primarily through two mechanisms:
- 1. Destroy the formation of the complex of PCSK9 and LDLR;
- 2. Directly inhibit the synthesis of PCSK9.
Monoclonal antibodies or imitated antibody proteins clear LDL-C by directly inhibiting the combination of PCSK9 and LDLR. At present, monoclonal antibodies are still the main field of PCSK9 inhibitors.
Small molecule peptide drugs can act on the catalytic site of PCSK9 protein, making it allosteric and then affecting the binding of PCSK9 and LDLR. MK-0616 developed by Merck & Co. (MSD), an oral small molecule PCSK9 inhibitor, is such a Small molecule cyclic peptides that can be synthesized and absorbed through the digestive tract are still in phase I clinical research.
In another mechanism-inhibition of PCSK9 synthesis, AZD8233, a PCSK9-targeted antisense oligonucleotide (ASO) drug jointly developed by AstraZeneca and Ionis, is used to target and inhibit the translation of PCSK9 mRNA in hepatocytes and protein synthesis. The same small interfering RNA (siRNA) also inhibits PCSK9 synthesis through gene silencing.
Leqvio: siRNA-based PCSK9 inhibitor
Novartis’ lipid-lowering drug, Inclisiran, with the trade name (Leqvio) was approved for marketing in the European Union in September 2020 and approved by the U.S. Food and Drug Administration (FDA) on December 13, 2021, becoming the world’s first RNAi-based drug.
It is an innovative injectable drug for cholesterol-lowering (LDL-C) therapy, and it is also one of the three PCSK9 inhibitors listed in the world.
After Leqvio enters hepatocytes, it binds to the RNA-induced silencing complex (RISC) and binds to the mRNA encoding PCSK9 protein under the mediation of the antisense strand, interfering with the transcription of specific genes that express a nucleotide sequence complementary to that of the After reducing the mRNA level of PCSK9, it prevents the expression of PCSK9 protein in the liver, thereby reducing the degradation of LDLR in lysosomes after the LDLR-PCSK9 complex is endocytosed, and enhancing the hepatic clearance of LDL from the blood by increasing the amount of LDLR in circulation -C ability to achieve the goal of lowering LDL-C levels.
In a double-blind, randomized, placebo-controlled, 18-month (540-day) phase III clinical trial, Leqvio was mainly included in the treatment of 3655 patients with heterozygous familial hypercholesterolemia (ORION-9), and in Outcomes in patients with atherosclerotic cardiovascular disease (ASCVD) (ORION-10) and patients with ASCVD or ACSVD equivalent risk (ORION-11) who required additional LDL-C lowering after statin therapy.
The primary endpoints of the study were the percent change in LDL-C from baseline to Day 510, and the time-adjusted percent change in LDL-C from Day 90 (baseline) to a maximum of Day 540.
All three studies met the primary endpoint. At the same time, the safety of the drug was also evaluated for 540 days. CeVD patients treated with Leqvio had a mean reduction in LDL-C of 55.2% from baseline to day 510 compared with placebo (P<0.0001).
Leqvio with two shots a year: Will it make the competition of PCSK9 inhibitors stronger?
In 2021, the sales revenue of Leqvio will reach 12 million US dollars (Figure 2). The initial performance of Leqvio is also very impressive compared with the listed Sanofi/Regeneron’s Praluent and Amgen’s Repatha. Because of better patient compliance with Leqvio:
Use the second injection 3 months after the initial treatment, and then two injections per year can control LDL cholesterol between 50-60%;
Both Praluent and Repatha require twice-monthly injections.
Analysts at GlobalData had predicted that Leqvio could earn Novartis $2.5 billion by 2027 for its ease of treatment, surpassing the projected sales of Praluent and Repatha by 2027.
At present, Leqvio is still conducting a trial testing cardiovascular therapy, which is expected to be completed in 2026. Whether Leqvio can be approved by the US FDA for cardiovascular indications will be our continued focus.

Figure 2. Approved 20+ drug pipeline products
Summary:
The statins that have implemented centralized drug collection can be said to be the most cost-effective treatment for cholesterol lowering. For adult patients with familial hypercholesterolemia, confirmed cardiovascular disease or statin intolerance, PCSK9 inhibitor single There is still some competitiveness in use or in combination with statins.
The research and development cost of monoclonal antibodies is the main reason for their high prices, which will inevitably increase the economic burden of patients; oral PCSK9 inhibitors have the best compliance, but their research and development process still has a long way to go. After all, oral preparations The low bioavailability of the drug is still an unavoidable problem, and its production cost will be higher than that of injections.
The current problems of high drug pricing, dosage form selection and bioavailability are common challenges faced by major pharmaceutical companies for the development of PCSK9 inhibitors.
References:
1.Erratum: Department of Error. (2018). Lancet (London, England), 392(10160), 2170. https://doi.org/10.1016/S0140-6736(18)32833-2
2.American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Executive Summary: Heart Disease and Stroke Statistics–2016 Update: A Report From the American Heart Association Circulation, 133 (2016), pp. 447-454
3.Duewell, P., Kono, H., Rayner, K. J., Sirois, C. M., Vladimer, G., Bauernfeind, F. G., Abela, G. S., Franchi, L., Nuñez, G., Schnurr, M., Espevik, T., Lien, E., Fitzgerald, K. A., Rock, K. L., Moore, K. J., Wright, S. D., Hornung, V., & Latz, E. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature, 464(7293), 1357–1361. https://doi.org/10.1038/nature08938
4.Singh, A., Gupta, A., Collins, B. L., Qamar, A., Monda, K. L., Biery, D., Lopez, J., de Ferranti, S. D., Plutzky, J., Cannon, C. P., Januzzi, J. L., Jr, Di Carli, M. F., Nasir, K., Bhatt, D. L., & Blankstein, R. (2019). Familial Hypercholesterolemia Among Young Adults With Myocardial Infarction. Journal of the American College of Cardiology, 73(19), 2439–2450. https://doi.org/10.1016/j.jacc.2019.02.059
5.Bekkering, S., Stiekema, L., Bernelot Moens, S., Verweij, SL, Novakovic, B., Prange, K., Versloot, M., Roeters van Lennep, JE, Stunnenberg, H., de Winther , M., Stroes, E., Joosten, L., Netea, MG, & Riksen, NP (2019). Treatment with Statins Does Not Revert Trained Immunity in Patients with Familial Hypercholesterolemia. Cell metabolism, 30(1), 1–2. https://doi.org/10.1016/j.cmet.2019.05.014
6.Seidah, N. G., & Prat, A. (2021). The multifaceted biology of PCSK9. Endocrine reviews, bnab035. Advance online publication. https://doi.org/10.1210/endrev/bnab035
Nassoury, N., Blasiole, D. A., Tebon Oler, A., Benjannet, S., Hamelin, J., Poupon, V., McPherson, P. S., Attie, A. D., Prat, A., & Seidah, N. G. (2007). The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic (Copenhagen, Denmark), 8(6), 718–732. https://doi.org/10.1111/j.1600-0854.2007.00562.x
7.Zhang, D. W., Garuti, R., Tang, W. J., Cohen, J. C., & Hobbs, H. H. (2008). Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proceedings of the National Academy of Sciences of the United States of America, 105(35), 13045–13050. https://doi.org/10.1073/pnas.0806312105
8.Horton, J. D., Cohen, J. C., & Hobbs, H. H. (2009). PCSK9: a convertase that coordinates LDL catabolism. Journal of lipid research, 50 Suppl(Suppl), S172–S177. https://doi.org/10.1194/jlr.R800091-JLR200
9.https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-therapy-lower-cholesterol-among-certain-h
10.Wright, R. S., Ray, K. K., Raal, F. J., Kallend, D. G., Jaros, M., Koenig, W., Leiter, L. A., Landmesser, U., Schwartz, G. G., Friedman, A., Wijngaard, P., Garcia Conde, L., Kastelein, J., & ORION Phase III Investigators (2021). Pooled Patient-Level Analysis of Inclisiran Trials in Patients With Familial Hypercholesterolemia or Atherosclerosis. Journal of the American College of Cardiology, 77(9), 1182–1193.
11. https://www.novartis.com.cn/news/nuo-hua-inclisiranzai-dong-mai-zhou-yang-ying-hua-xing-xin-xie-guan-ji-bing-%28ascvd%29ya -zu-zhon-0
12.Novartis’ Leqvio to reap $2.5B by 2027 thanks to convenience edge over Amgen, Sanofi rivals: analyst
Will PCSK9 inhibitors become the main lipid-lowering drugs in the future?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



