Sorrento develops RBD chimeric COVID-19 mRNA vaccine
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
- New Ocrevus Subcutaneous Injection Therapy Shows Promising Results in Multiple Sclerosis Treatmen
- Dutch Man Infected with COVID-19 for 613 Days Dies: Accumulating Over 50 Virus Mutations
Sorrento develops RBD chimeric COVID-19 mRNA vaccine
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Sorrento develops RBD chimeric COVID-19 mRNA vaccine.
On March 7, 2022 , Sorrento Theraputics submitted a preprint on BioRxiv : Chimeric mRNA based COVID-19 vaccine induces protective immunity against Omicron and Delta , researchers mutated the S1/S2 furin cleavage site to design a series of new coronavirus VOCs Spike mRNA vaccine for better immunity.
On this basis, the researchers chimeric Delta-RBD into the Omicron Spike mRNA backbone, located upstream of the Omicron RBD, to create a chimeric Spike-furin mRNA with Omicron Spike as the backbone and tandem Delta-RBD and Omicron RBD.
The vaccine has a wider spectrum of serum neutralizing antibodies, especially the serum neutralizing antibody titer targeting the Detla variant, which provides new ideas for optimizing the COVID-19 mRNA vaccine.
Cause of S1/S2 furin cleavage site mutation
The new coronavirus Spike protein monomer consists of an S1 recognition domain and an S2 fusion domain .
There is a furin enzyme cleavage site at the junction of S1 and S2, and the complete Spike protein exists in the form of an inactive precursor. When RBD binds to host cell ACE2, furin cleaves S1/S2 , and then the exposed S2 subunit mediates fusion with the host membrane.
There are S1/S2 furin cleavage sites in the Spike proteins of the two approved mRNA vaccines. The existence of S1/S2 furin cleavage sites will affect the stability of the Spike protein expressed by mRNA vaccines and reduce the risk of triggering adaptive immune responses. epitope library.
Some studies have found that mRNA1273 vaccine recipients can detect the presence of S1 in serum 1-5 days after the first vaccination , which may be caused by protease cleavage in mammalian cells or in the circulatory system.
In addition, many vital organs can take up S1, such as the spleen, kidney, liver, and even cross the blood-brain barrier to the brain.
Since S1 contains a complete RBD, it can still bind to ACE2, triggering a series of downstream signaling events, which may lead to inflammation and lung diseases.
Although it is not clear whether S1 will cause clinical consequences, in order to eliminate the potential safety hazards caused by the S1/S2 furin cleavage site, when designing and optimizing new mRNA vaccines, researchers mutated the S1/S2 furin cleavage site. .
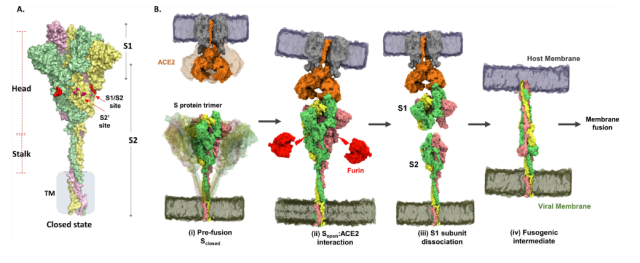
The new coronavirus Spike protein recognizes the host cell ACE2 and triggers the membrane fusion process
Furin enzyme cleavage site mutation increases full-length Spike content
The researchers designed two groups of mRNA vaccines, one encoding the VOC new coronavirus wild-type Spike-WT , and the other encoding the VOC new coronavirus mutant Spike-furin .
Flow data showed that removal of the S1/S2 furin cleavage site increased full-length Spike on the surface of transfected cells.
WB also confirmed that after the furin cleavage site was eliminated, the Spike-furi mRNA encoded in the transfected cells produced the full-length Spike protein , while the Spike-WT mRNA was detected in the cells or in the medium after transfection of the cells. to S1.
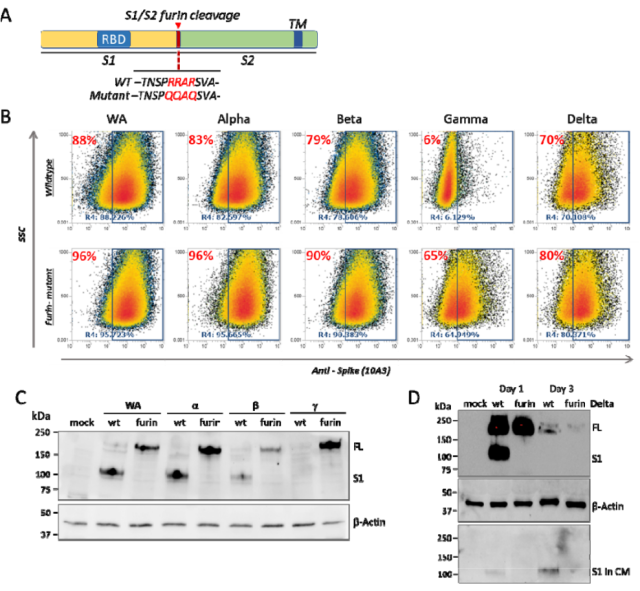
Spike-furin mRNA transfects cells to encode full-length Spike
Immune Response Triggered by Spike-furi mRNA Vaccine
The researchers wanted to know whether the increase in the full-length Spike protein content caused by the furin site mutation would enhance the immune response, and injected the new coronavirus wild strain Spike-WT-mRNA vaccine and Spike-furi mRNA vaccine into mice.
It was found that the Spike-furi mRNA vaccine could trigger higher serum binding antibody titers and neutralizing antibody titers.
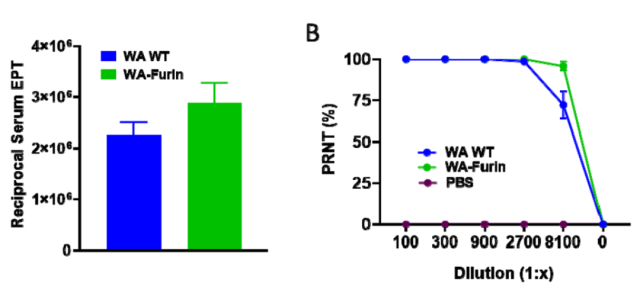 Differences in the titers of binding and neutralizing antibodies in mouse serum elicited by 2019-nCoV wild strain Spike-WT mRNA and Spike-furin mRNA
Differences in the titers of binding and neutralizing antibodies in mouse serum elicited by 2019-nCoV wild strain Spike-WT mRNA and Spike-furin mRNA
The researchers constructed various new coronavirus VOC Spike-furin mRNA vaccines and immunized mice.
The results found that, except for the Gamma Spike-furin mRNA vaccine, the serum-binding antibodies triggered by other new coronavirus VOC Spike-furin mRNA vaccines had the highest titers. source serum antibodies .
Notably, some VOC Spike-furin mRNA vaccines elicited serum antibodies with broader binding capacity, such as the Beta-Spike-furin mRNA vaccine.
The neutralization test data of five VOC live viruses showed that the neutralization activity of serum antibodies triggered by each new coronavirus VOC Spike-furin mRNA vaccine was generally strain-specific .
For example, WA-Spike-furin mRNA vaccinated mice elicited the highest neutralizing activity of serum neutralizing antibodies against the WA original strain.
At the same time, it can also be observed that the serum neutralizing antibodies triggered by the Beta-Spike-furin mRNA vaccine have a broader and stronger neutralizing ability .
In particular, the neutralizing activity of serum neutralizing antibodies triggered by the Beta-Spike-furin mRNA vaccine against the Delta strain was much stronger than that triggered by the WA-Spike-furin mRNA vaccine .
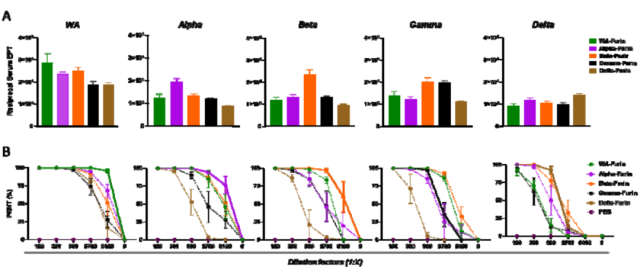 Differences in serum antibody titers between homologous and heterologous mice induced by novel coronavirus VOC Spike-furin mRNA vaccine
Differences in serum antibody titers between homologous and heterologous mice induced by novel coronavirus VOC Spike-furin mRNA vaccine
The researchers constructed WA Spike-furin mRNA vaccine and Beta-Spike-furin mRNA vaccine, injected different doses of mRNA vaccine, isolated spleen cells, and stimulated with S1 antigen peptide.
TH1 response ( characterized by secreted IFNγ and TNF ), very weak TH2 response ( characterized by secreted IL-4 and IL-5 ). However, the two vaccines triggered very small changes in the number of CD+4 cells in the spleen, the number of CD8+ cells, and the number of memory B cells in the serum and spleen.
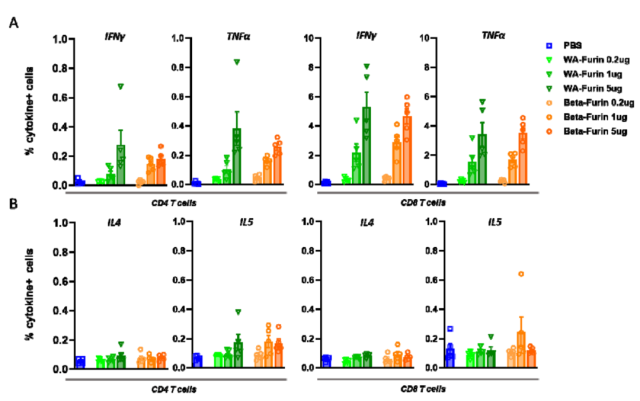 TH1 and TH2 responses triggered by Spike-furin mRNA vaccine
TH1 and TH2 responses triggered by Spike-furin mRNA vaccine
The protective effect of Spike-furin mRNA vaccine
The researchers injected mice with 2 doses of WA Spike-furin mRNA vaccine / Beta-Spike-furin mRNA vaccine respectively , with an interval of 3 weeks, and conducted a challenge test in the 8th week to observe the changes in the lung viral load and weight changes.
Compared with the unvaccinated group, the mice vaccinated with the two vaccines had no detectable virus in the lungs and no weight loss , indicating that both vaccines can trigger a very strong immune protection effect.
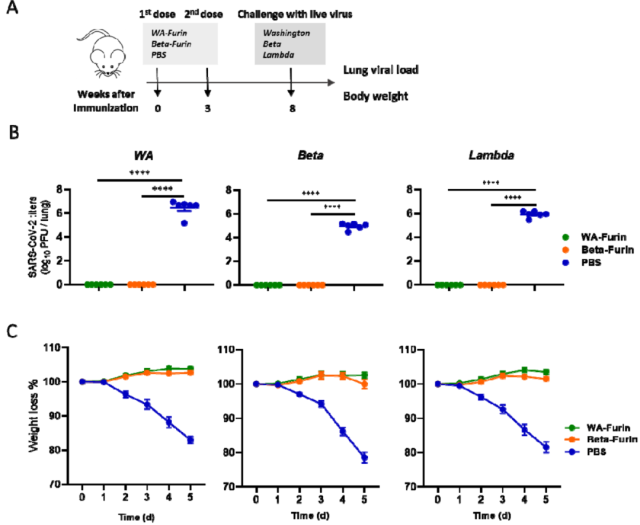 Inoculation of mice with Spike-furin mRNA vaccine triggers strong preventive protection
Inoculation of mice with Spike-furin mRNA vaccine triggers strong preventive protection
Omicron weakens the immune effect of furin mRNA vaccine
The researchers found that 14 days after inoculation of the second dose of WA Spike-furin mRNA vaccine, the serum neutralizing antibody titer IC50 targeting the Omricon variant strain was only 318 , compared with the serum neutralizing antibody titer targeting the original strain WA of 16914 , 53 times lower .
The serum neutralizing antibody titers of mice vaccinated with other types of VOC Spike-furin mRNA vaccines also decreased significantly when targeting the Omricon variant.
Even the Beta Spike-furin mRNA vaccine, which triggered broader neutralizing antibody activity in previous trials, failed to provide adequate protection against the Omricon variant.
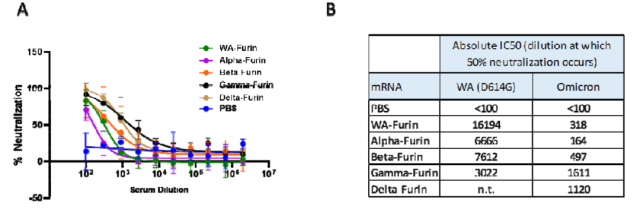 Serum antibody neutralization activity of targeting Omicron variants triggered by vaccination with different SARS-CoV-2 VOC Spike-furin mRNA vaccines
Serum antibody neutralization activity of targeting Omicron variants triggered by vaccination with different SARS-CoV-2 VOC Spike-furin mRNA vaccines
Omicron Spike-furin mRNA Vaccine
In order to develop a more efficient vaccine targeting Omicron, the researchers constructed the Omicron Spike-furin mRNA vaccine, and found that the serum binding antibody titer and neutralizing antibody titer of the Omicron variant targeting the Omicron variant were both in mice vaccinated with the Omicron Spike-furin mRNA vaccine. was significantly improved and triggered a significant dose-dependent immune cell response .
The data of the challenge test with Omicron variant strains showed that no virus replication was detected in the lungs regardless of 2 doses of homologous WA Spike-furin mRNA vaccine or the heterologous boost of Omicron Spike-furin mRNA vaccine
. However, through yesterday’s article CureVac/GSK published preclinical data of bivalent mRNA COVID-19 vaccine , we learned that it is very difficult to inhibit the replication of new coronavirus VOCs in the upper respiratory tract, so the absence of virus replication in the lungs here does not fully characterize immunity.
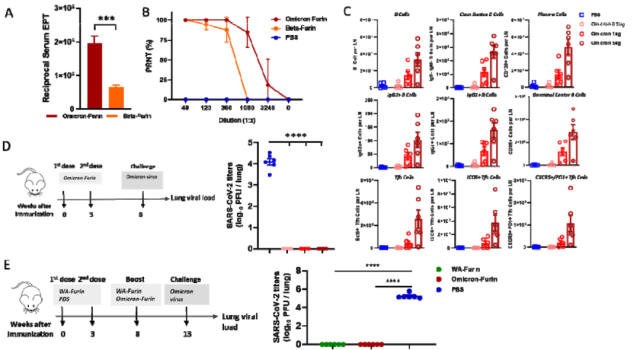 Omicron Spike-furin mRNA Vaccine Triggers Strong Immune Protection Against Omicron Variants
Omicron Spike-furin mRNA Vaccine Triggers Strong Immune Protection Against Omicron Variants
RBD chimeric mRNA vaccine
The researchers found that the Omicron Spike-furin mRNA vaccine had very limited .
In order to develop an mRNA vaccine with broad protective effect against other VOC variants, researchers placed Delta-RBD upstream of Omicron RBD in Omicron Spike, and constructed a tandem Delta-RBD and Omicron RBD embedded with Omicron Spike as the backbone. Hybrid Spike-furin mRNA vaccine.

Compared with the Omicron Spike-furin mRNA vaccine, mice immunized with the Delta -RBD/Omicron RBD chimeric Spike-furin mRNA vaccine significantly increased the serum-binding antibody titers targeting WA, Beta, and Gamma .
Changes in serum binding antibody titers for Alpha and Delta were not statistically different . Mice vaccinated with Omicron Spike-furin mRNA vaccine and Delta-RBD/Omicron RBD chimeric Spike-furin mRNA vaccine targeting Omicron BA.1 , Omicron R346K , Omicron BA.2 had similar neutralizing serum antibody titers , but, Compared with the Omicron Spike-furin mRNA vaccine, vaccination with the chimeric Spike-furin mRNA vaccine can significantly increase the serum neutralizing antibody titers targeting the Delta strain .
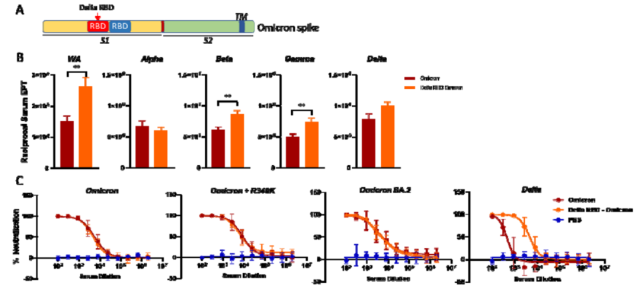 Broad spectrum of significant serum neutralizing antibodies of the Delta-RBD/Omicron RBD chimeric Spike mRNA vaccine
Broad spectrum of significant serum neutralizing antibodies of the Delta-RBD/Omicron RBD chimeric Spike mRNA vaccine
Summary
Different new coronavirus VOCs have different Spike protein mutations and thus different antigenic epitope libraries.
The highest titers of neutralizing antibodies in serum triggered by vaccination with different VOCs Spike mRNA vaccines were isotype neutralizing antibodies .
For example, the highest titers of neutralizing antibodies in serum triggered by vaccination with Delta Spike mRNA vaccines were those targeting Delta variants. , rather than targeting other VOCs. Notably, WA/Beta Spike mRNA vaccines, especially /Beta Spike mRNA vaccines, triggered serum-neutralizing antibodies that provided broader neutralization of VOCs .
This may be due to Beta Spike protein mutations, especially in the RBD and NITD regions, which are also present in other VOCs, such as L18, K417, E484, N501, D614. The Phase II clinical data of the mRNA 1273.211 bivalent vaccine recently announced by Moderna is also the other half of the selection of Beta Spike-mRNA as the bivalent vaccine.
This study uses 2 strategies to optimize the COVID-19 mRNA vaccine:
- The first is to mutate the S1/S2 furin cleavage site , which eliminates safety concerns, retains full-length Spike in cells and on the cell surface, provides a more presented epitope pool, and better triggers specific immune responses. In fact, in our previous article on the immunogenicity of different mutant RBD mRNA vaccines in mice, researchers at Oxford University also mutated the S1/S2 furin cleavage site to construct the Omicron-Spike mRNA vaccine Time.
- What is more innovative is another strategy, using Omicron Spike mRNA as the backbone and embedding Delta RBD upstream of Omicron RBD to construct a Delta-RBD/Omicron RBD chimeric Spike-furin mRNA vaccine, which can improve the broad spectrum of serum antibodies , in particular, significantly increased serum neutralizing antibody titers targeting the Delta variant . Our previously published article COVID-19 | Immunogenicity of different mutant RBD mRNA vaccines in mice , some researchers have hybridized the mutation sites of Delta RBD and OmicronRBD regions, but the hybrid Delta + Omicron- RBD mRNA vaccine significantly improves the target Except for the serum neutralizing antibody titers of the Omicron variant, the serum neutralizing antibody titers targeting other VOCs were not improved, and the serum neutralizing antibody effect was much weaker than that of the Delta-RBD+Omicron RBD bivalent mRNA vaccine .
This RBD chimeric strategy or the direct Spike/RBD mRNA bivalent or multivalent vaccine strategy , or the Spike+RBD mRNA vaccine strategy , provides a variety of options for upgrading the COVID-19 mRNA vaccine.
On the road to building a broad-spectrum mRNA vaccine , what kind of strategy can achieve both cost and immune effect requires further exploration.
This kind of exploration is not only beneficial to the upgrade and optimization of the COVID-19 mRNA vaccine, but also has reference significance for the research and development of other infectious diseases or tumor vaccines.
Sorrento develops RBD chimeric COVID-19 mRNA vaccine
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



