The results of the first clinical trials of the Moderna Omicron version vaccine are announced
- EPA Announces First-Ever Regulation for “Forever Chemicals” in Drinking Water
- Kochi University pioneers outpatient bladder cancer treatment using semiconductor lasers
- ASPEN 2024: Nutritional Therapy Strategies for Cancer and Critically Ill Patients
- Which lung cancer patients can benefit from neoadjuvant immunotherapy?
- Heme Iron Absorption: Why Meat Matters for Women’s Iron Needs
- “Miracle Weight-loss Drug” Semaglutide Is Not Always Effective
The results of the first clinical trials of the Moderna Omicron version vaccine are announced
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The results of the first clinical trials of the Moderna Omicron version vaccine are announced: The immune response is broader and the safety remains unchanged.
The FDA will discuss on June 28 whether to refer to the flu vaccine and use an updated version of the COVID-19 vaccine in the fall and winter to better deal with the mutant strains circulating today.
This plan is mainly due to the fact that the currently circulating mutant strains are all branches of Omicron, and compared with the original virus strain, there are many mutations in the key antigenic spike protein, and the existing vaccine designed with the original virus strain as the blueprint Shows strong immune escape .
For example, the same two or three injections of mRNA vaccine, the neutralizing antibody to Omicron is much lower than the original virus strain:
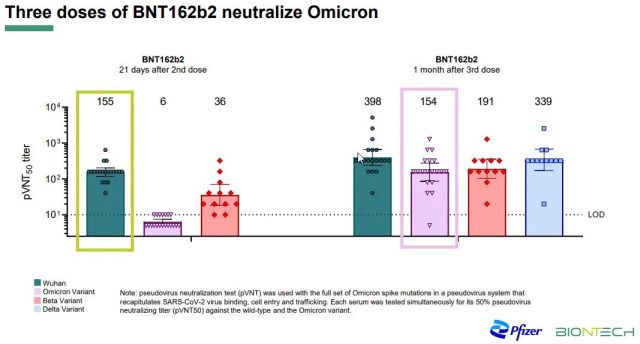
Note that although the neutralization ability of Omicron has been greatly improved after the third injection (enhanced injection), it still has a big gap compared with the original virus strain.
This immune escape is also reflected in the effectiveness of the vaccine against various branches of Omicron, especially the lack of effectiveness in preventing infection or mild infection. If the vaccination time is longer, the decline will be very fast.
In response to the immune escape caused by Omicron due to many mutations, the natural idea is to make the vaccine closer to the “look” of Omicron .
Make an Omicron version of the vaccine, and the immune response to Omicron may be better than the original vaccine.
At the same time, considering that the antigen recognition position occupied by Omicron is far away from the previous mutants [1]:
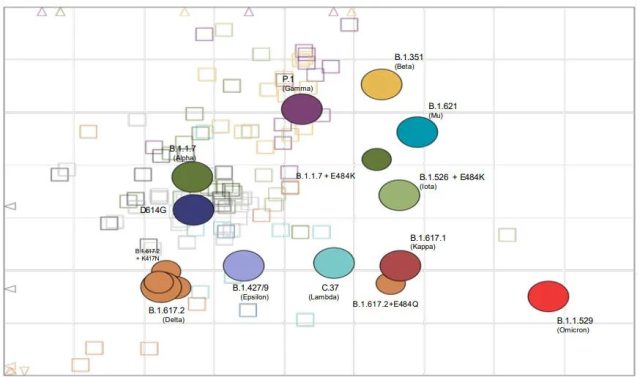
In order to make the immune recognition range wider and prevent Omicron from losing the recognition of other virus strains (including the original virus strain), the safest approach is multivalent design .
That is, a vaccine has both the original virus strain or a similar design, and the Omicron version. Such multivalent vaccines may induce a broader spectrum of immune protection and be more secure in the face of unpredictable COVID-19 mutation directions.
These theoretical assumptions are all reasonable, but the problem is that we still need evidence: the Omicron vaccine, or a multivalent vaccine containing the Omicron vaccine, is indeed better than the original vaccine – immunity to Omicron The response was stronger, but also broad enough (not worse for other mutants).
Such evidence has only been obtained through clinical trials of the Omicron version of the vaccine.
A number of pharmaceutical companies including Pfizer/BioNTech and Moderna have also launched clinical trials of the Omicron version of the vaccine, which will begin as early as the end of January 2022.
Today (June 8, 2022), Moderna finally announced the results of the first clinical trial of a new-generation COVID-19 vaccine containing the Omicron vaccine, showing that the bivalent design using Omicron/original virus strain is relatively The original vaccine, as a booster, induced a better immune response to Omicron , while a similar immune response to the original strain [2].
The result comes from a Phase 2/3 clinical trial of Moderna. The subjects were all vaccinated with three doses of Moderna’s original vaccine, mRNA-1273, which was 100 micrograms for the first two injections and 50 micrograms for the third injection.
In the test, subjects were divided into 1:1 groups and were respectively vaccinated with a booster shot of the original vaccine (377 people) or a booster shot of the bivalent vaccine (437 people), both at a dose of 50 micrograms.
The difference is that mRNA-1273.214 includes 25 micrograms of the original vaccine and 25 micrograms of the Omicron version vaccine (based on BA.1 design, with 32 mutations compared to the original virus spike protein) . Subject characteristics are as follows [3]:
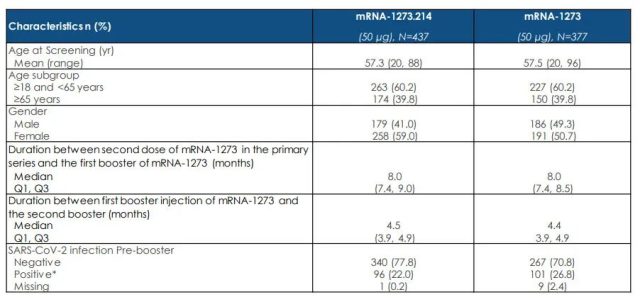
As can be seen from the table, 40% of the subjects were aged over 65, and more than 20% had a history of past infection. Such a “diverse” population means the results will be generalizable.
The mRNA-1273 group was enrolled from February 21st to March 8th, and the mRNA-1273.214 group was enrolled from March 8th to 23rd.
Judging from the time of enrollment, it should meet the commonly used safety tracking time standard for emergency use authorization of vaccines, which is a median follow-up of 2 months after vaccination.
It can also be used as a reference to speculate about how long it will take for those who have recently started the Omicron version of the vaccine trial to get meaningful results.
Since there were only about 400 people in each group, the trial did not aim to compare the differences in COVID-19 infection after vaccination between the two vaccines, but to compare indicators of immune response , such as neutralizing antibodies. If mRNA-1273.214 induces significantly higher neutralizing antibodies than the original mRNA-1273, then the new bivalent design is superior in immune response to the original design and may also correspond to better protection. Neutralizing antibody titer tests by Omicron demonstrate this [3]:
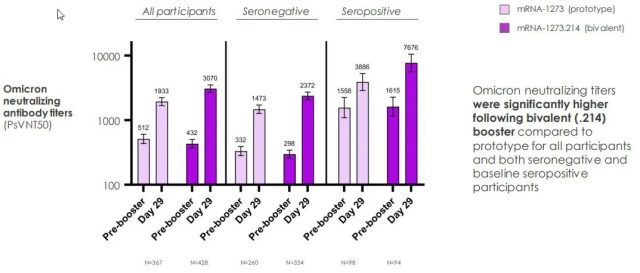
One month after inoculation, the neutralizing antibody titer of Omicron in the mRNA-1273.214 group was significantly higher than that in the mRNA-1273 group , regardless of whether there was a history of past infection.
Based on the analysis of all testers, the average neutralizing antibody titer of the mRNA-1273.214 group was 3232.52, which was 1.78 times the neutralizing antibody titer of the original vaccine group of 1815.14 :
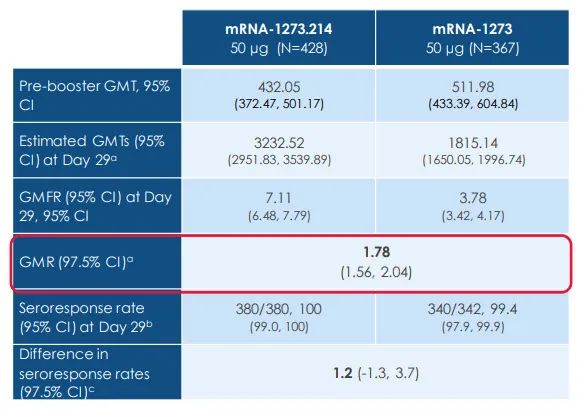
If we only look at people with no past infection history, the neutralizing antibody titer of the mRNA-1273.214 group is still 1.72 times that of the original vaccine group.
If you look at the neutralizing antibody titer against the original virus strain [3]:
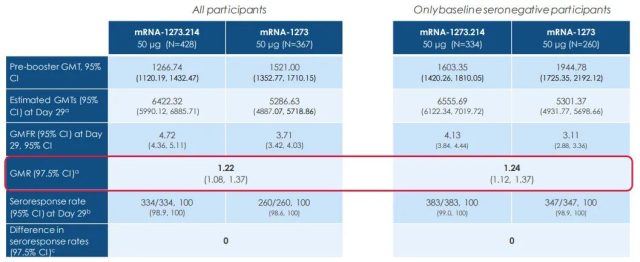
The neutralizing antibody titer of the mRNA-1273.214 group against the wild-type virus was about 1.2 times that of the original vaccine group , no worse.
That is to say, using the bivalent (Omicron + original strain) vaccine design, the immune response of mRNA-1273.214 against Omicron is better than the existing vaccine, and the immune response against the original virus strain is no worse than the existing vaccine .
In terms of adverse reactions, mRNA-1273.214 seems to have a lesser trend than the second and third doses of the original vaccine, but it is basically similar [3]:
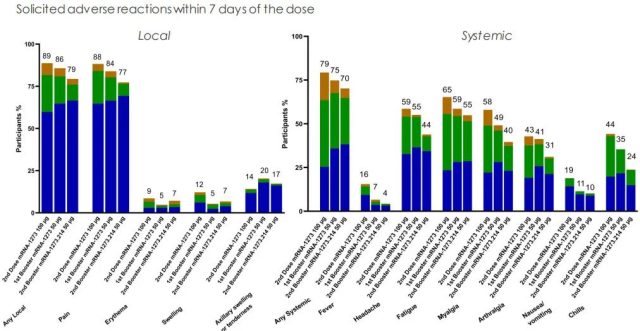
Overall, this result shows that mRNA-1273.214 achieved a broader immune response than the original vaccine through bivalent design, more neutralizing antibodies to Omicron, and was similar in safety to the original vaccine.
This provides key support for the Omicron version of the vaccine, and also provides a very important reference for the design of the next-generation COVID-19 vaccine.
Moderna has submitted relevant research results to regulatory agencies, expecting the bivalent COVID-19 vaccine of the Omicron/original virus strain to be available in late summer this year, in preparation for the autumn and winter booster shots.
In fact, the results are likely to affect all COVID-19 vaccine designs in most parts of the world.
Not surprisingly, the FDA will refer to the mutant vaccine research of multiple vaccine manufacturers, including the Moderna trial, to unify the design of the COVID-19 vaccine.
The bivalent design of the Omicron+ original virus strain is very likely to become the “season change” standard .
The results for mRNA-1273.214 met all of our requirements for a replacement vaccine—broader-spectrum immune response with no change in adverse effects.
But its actual utility still needs to hold rational expectations.
Higher neutralizing antibodies to Omicron may correspond to better efficacy, but how big is the actual effect? Can it stop the infection? It’s hard to say.
After all, the neutralizing antibody to Omicron is not low in a short period of time after the three injections of the original mRNA vaccine, and there are still many people infected.
Realistically speaking, mRNA-1273.214 will still be a vaccine that mainly prevents severe illness and death, rather than absolutely preventing infection .
The higher neutralizing antibodies to Omicron may have more of a role in the slower decline in effectiveness over time.
A broader immune response could also make the vaccine’s effectiveness less hit in the event of another mutant strain.
Today, the most important mutant strains in the United States are no longer BA.1 designed by mRNA-1273.214, but BA.2.12.1, which also has strong immune escape to BA.1, and BA that also has significant immune escape to BA.1. .4/5 is also eyeing.
In the autumn and winter when the replacement vaccines such as mRNA -1273.214 are on the market, it is hard to say what the popular mutants will be.
Therefore, from a rational point of view , although the replacement of mRNA-1273.214 is stronger than no replacement, it will not cause earth-shaking changes in the effectiveness, effect and impact of the epidemic.
The actual vaccine replacement also has many details for regulators to consider. For example, what Moderna has published is only the results of a second booster shot in adults.
From the perspective of reducing confusion, the initial vaccination, the first booster shot and the juvenile vaccine should be replaced together, but do these need more experimental evidence?
From the perspective of execution, in addition to Moderna, Pfizer/BioNTech will also have Omicron version vaccine data with high probability to catch up with the FDA meeting on June 28, but for Novavax and Novavax, which have just started the Omicron version vaccine trial, and are downgraded to the second The Johnson & Johnson vaccine used at the first level is not easy to say.
Will every manufacturer be required to do a similar level of testing of the Omicron version of the vaccine?
Or simplify the requirements to ensure that there are multiple vaccines on the market with different technology platforms (a point repeatedly emphasized by the FDA in its review of the Novavax vaccine)?
These problems may not have perfect solutions, only trade-offs that must be made.
Whether it is a childhood vaccine (the vaccination rate of the 5-11-year-old vaccine is stagnant at 30% for half a year), or the first booster shot (the vaccination rate among those who meet the standard is stagnant at 50%), the problem encountered in the United States is not that the vaccine is not effective enough , The security is not good, but the acceptance is too low .
With each crowd expansion, the acceptance is getting lower and lower.
Therefore, in the end, no matter how the vaccine is replaced, the key to actual success will be how to improve the public’s acceptance of the next vaccine without compromising scientificity.
References: The results of the first clinical trials of the Moderna Omicron version vaccine are announced
1. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-april-6-2022-meeting-announcement
2. https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-Omicron-Containing-Bivalent-Booster-Candidate-mRNA-1273.214-Demonstrates-Superior-Antibody-Response-Against-Omicron/ default.aspx
3. https://s29.q4cdn.com/435878511/files/doc_presentations/2022/06/08/Master-Final-Bivalent-Omicron-Data-Update-0608.pdf
The results of the first clinical trials of the Moderna Omicron version vaccine are announced
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



