PD-1 immune combination therapy for gastric cancer
- Engineered Soybeans with Pig Protein: A Promising Alternative or Pandora’s Dish?
- Severe Fever with Thrombocytopenia Syndrome (SFTS): A Tick-Borne Threat with High Mortality
- Why Isolating Bananas Extends Their Shelf Life?
- This common vitamin benefits the brain and prevents cognitive decline
- New report reveals Nestlé adding sugar to infant formula sold in poor countries
- Did Cloud Seeding Unleash a Deluge in Dubai?
PD-1 immune combination therapy advances to first-line treatment of gastric cancer
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
PD-1 immune combination therapy advances to first-line treatment of gastric cancer.
Immunotherapy is rewriting the treatment landscape for gastric/esophagogastric junction adenocarcinoma (G/GEJAC), a drug that treats BMS and MSD in the Big Three thanks to its PD-1 therapy, while another benefits from Roche in the HER2-targeted therapy Herceptin.
How to choose a combination strategy and explore other special population drug treatments are the next issues facing the clinical development of drugs in this market.
Increased use of immunotherapy
Due to the limited efficacy of immunotherapy alone in gastric cancer, it is currently used in combination with chemotherapy or targeted therapy to improve the efficacy.
Nivolumab and Pembrolizumab have led the way in first-line treatment of HER2-negative and positive gastroesophageal cancer, respectively.
In April 2021, Nivolumab in combination with chemotherapy was approved for first-line treatment of HER2-negative advanced gastric and esophageal cancer, regardless of PD-L1 expression.
Then in May, Pembrolizumab combined with Trastuzumab and chemotherapy was approved for first-line treatment of HER2-positive advanced gastric and esophageal cancer.
Meanwhile, in July, Pembrolizumab was voluntarily withdrawn as a single agent for third-line treatment of PD-L1-positive gastric cancer.
On May 28, 2022, BMS took the lead in winning the first-line treatment indication for HER2-negative advanced esophageal squamous cell carcinoma, shifting from chemotherapy alone to dual immunotherapy.
The US FDA approved two nivolumab-based regimens (in combination with fluoropyrimidine- and platinum-containing chemotherapy; in combination with the anti-CTLA-4 antibody ipililumab).
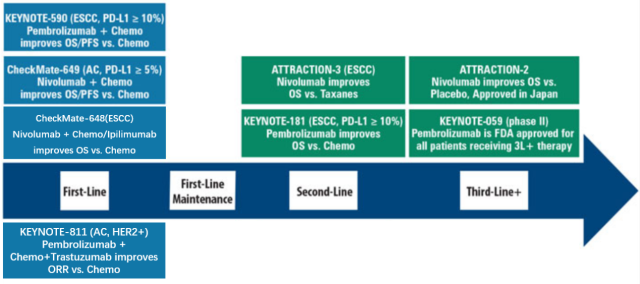
Immunotherapy enters first-line therapy for gastric and esophageal cancer
Other PD-1 antibodies, including Tislelizumab, are also undergoing phase III trials in combination with chemotherapy for first-line treatment of patients with HER2-negative G/GEJAC.
Combination strategies are not only used with chemotherapy, but also with anti-angiogenesis and other immune-related targets are becoming more and more important.
Pembro is combining with VEGFR inhibitor lenvatinib + chemotherapy as first-line treatment of G/GEJAC. Phase III trial NCT04662710).
Nivolumab is in a phase III trial in combination with Relatlimab (LAG-3 antibody) + chemotherapy as first-line treatment for G/GEJAC.
When the first-line treatment landscape has changed, the clinical development of subsequent products also faces problems.
The first is the choice of chemotherapeutic drugs. Chemotherapy is still the basis for combined exploration of first-line treatment. The current research on chemotherapy is more inclined to use better chemotherapy options than chemotherapy alone.
In the KEYNOTE-590 trial, Pembrolizumab was tested in combination with cisplatin + FU, but cisplatin is no longer the preferred first-line chemotherapy for gastric cancer; and Nivolumab combined with XELOX or FOLFOX is currently a better choice for first-line chemotherapy.
The second is the choice of the control group. One of the main problems faced by late-stage pipeline drugs in the phase III confirmatory trial is that the first-line standard treatment has changed.
Immunotherapy has become the first-line standard treatment for patients with HER2-positive or negative gastric cancer.
The control group in the HER2-negative population still chooses chemotherapy alone, and the latecomers may need to prove whether they are really more advantageous than first-line immunotherapy when they really seek marketing approval.
HER2-positive new therapy
About 20% of G/GEJAC patients are HER2 positive. For HER2-positive patients, the current efficacy of HER2-targeted drugs alone is not good.
The main strategy for development is to use new mechanisms of action or add combinations to improve efficacy.
ADC is one of the strategies. The HER2-targeting ADC drug T-DXd (trastuzumab deruxtecan) jointly developed by Daiichi Sankyo and AstraZeneca has been approved by the FDA for the treatment of patients with locally advanced or trastuzumab-based regimens.
Metastatic HER2-positive G/GEJAC patients. Based on the randomized pivotal DESTINY-Gastric01 phase II trial, patients treated with T-DXd (n=126) had a 41% lower risk of death and a median OS of 12.5 months compared to those receiving chemotherapy (n=62) vs. chemotherapy 8.4 months.
The excellent efficacy of T-DXd in the first-line treatment of metastatic breast cancer with low HER2 expression may also accelerate the acquisition of this indication.
Dual HER2 targeting is also one of the strategies. The bispecific antibody zanidatamab developed by Zymeworks can dual block HER2 signaling, increase the clearance of HER2 protein from the cell surface, and improve antibody-mediated cytotoxicity.
Zanidatamab combined with anti-PD-1 antibody tislelizumab and CAPOX chemotherapy regimen was used as a first-line treatment trial for HER2-positive G/GEJAC, with ORR of 75.8%.
The disease control rate was 100%, and the duration of response ranged from 2.1 months to more than 18.2 months. Currently this combination can compete with Pembro + trastuzumab + chemotherapy triple therapy.

Zanidatamab+tislelizumab+chemotherapy for gastric cancer first-line phase I/II trial results
To date, no HER2-targeted therapy has shown better OS than chemotherapy as a second-line therapy, which may be due to the loss of HER2 expression following trastuzumab-based therapy.
Tucatinib, a small molecule HER2 inhibitor developed by Seagen, is undergoing a phase II/III trial called MOUNTAINEER-02 (NCT04499924), evaluating the quadruple therapy of Ramucirumab + Paclitaxel + Tucatinib + Trastuzumab versus Ramucirumab + Paclitaxel as second-line therapy Efficacy.
In addition to HER2 itself, other targets are being used in combination with one or both of trastuzumab and/or Cyramza.
This includes ALX Oncology’s evorpacept (a CD47 inhibitor), which is being studied in combination with trastuzumab and ramucirumab as a second-line treatment for HER2-positive gastric cancer, with an ORR of 72% in a phase Ib study.
Other specific mutation types
For HER2-negative patients, new drugs targeting specific patient subsets have also become popular, including FGFR2, CLDN18.2, etc.
FGFR2b is amplified and overexpressed in 5% to 10% of patients with gastroesophageal adenocarcinoma, and 30% of HER2-negative gastric cancers are positive for FGFR2b.
The discovery of this target was very early, and there are many tyrosine kinases covering this target, but it did not show confirmed efficacy in gastric cancer until Bemarituzumab became famous in a trial called FIGHT, which may lead to This old target entered the golden age of gastric cancer.
Bemarituzumab is a FGFR2b receptor-specific IgG1 antibody that selectively blocks FGFR2b-mediated growth factor signaling.
In the phase II FIGHT trial of bemarituzumab combined with mFOLFOX6 chemotherapy versus chemotherapy in patients with HER2-negative, FGFR2B-positive gastric adenocarcinoma, the bemarituzumab group showed a survival benefit with a PFS of 9.5mos vs. placebo 7.4mos and a lower risk of death than the placebo group 42% (not reached vs. 12.9mos, HR=0.58, p=0.027). A registrational phase III trial is also underway based on this result.
Ongoing trial of bemarituzumab in first-line gastric cancer
About 30% of HER2-negative patients are considered to have more than 70% of tumor cells expressing CLDN18.2, especially in gastric and pancreatic cancer, the fastest progress is Astellas Pharmaceutical’s CLDN18.2 monoclonal antibody zolbetuximab, which is currently being combined with mFOLFOX6 Phase III trial of combination versus mFOLFOX6 as first-line therapy for gastric adenocarcinoma.
In addition to monoclonal antibodies, many products choose to develop ADCs or double antibodies against other T cell targets.
Many companies are rushing to deploy in this target, and cooperation outside of self-research has also increased significantly.
PD-1 immune combination therapy advances to first-line treatment of gastric cancer.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



