Famous papers questioned: The loss of Ptbp1 could not induce the transformation of glial cells into neurons
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Famous papers questioned: The loss of Ptbp1 could not induce the transformation of glial cells into neurons
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Famous papers questioned: The loss of Ptbp1 could not induce the transformation of glial cells into neurons.
Replenishing cells lost to disease is the holy grail of regenerative medicine, yet the central nervous system (CNS) of adult mammals , including humans , has largely lost this regenerative capacity.
In recent years, an emerging strategy has held great promise for treating neurodegenerative diseases by converting glial cells in vivo into neurons. But trying to achieve this is not only complicated but also inefficient.
In 2020, two top journal papers published in Cell and Nature respectively showed that just knocking down Ptbp1 can efficiently induce the transformation of glial cells into neurons and rescue the motor impairment of the Parkinson’s disease mouse model. This holds great promise for the treatment of neurodegenerative diseases through glial-to-neuron transdifferentiation.
However, other groups have not been able to replicate the results of these two papers, and people have begun to question whether the conversion of glial cells to neurons really occurs after knocking down Ptbp1?
In April 2020, Yang Hui ‘s team from the Institute of Neurology, Chinese Academy of Sciences published a paper titled: Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice in the journal Cell [1] . The study found that knocking down Ptbp1 using the CRISPR-CasRx system can induce the transformation of glial cells into neurons.
In June 2020, Fu Xiangdong ‘s team at the University of California, San Diego published a paper in Nature entitled: Reversing a model of Parkinson’s disease with in situconverted nigral neurons [2] . This study found that knockdown of Ptbp1 protein expression using shRNA or antisense oligonucleotides (ASO) could induce the transformation of glial cells into neurons.
These two studies suggest that the Ptbp1 gene may be a potential target for the treatment of neurodegenerative diseases caused by neuronal damage, including Parkinson’s disease.
After these two papers were published, they aroused widespread concern and controversy in the industry.
In September 2021, Zhang Chunli’s team at the University of Texas Southwestern Medical Center published a paper titled: Revisiting astrocyte to neuron conversion with lineage tracing in vivo in Cell [ 3] .
The study used lineage tracing to demonstrate that neither overexpressing NeuroD1 nor knocking down Ptbp1 could convert glial cells into neurons in vivo, and that the transdifferentiated neurons observed in previous studies were actually neurons in the brain. Pre-existing endogenous neurons.
In October 2021, the Seth Blackshaw team of Johns Hopkins University School of Medicine published a paper entitled: Ptbp1 deletion does not induce glia-to-neuron conversion in adult mouse retina and brain in the preprint bioRxiv [4] .
The paper noted that specific deletion of Ptbp1 did not induce glial-to-neuron conversion in both the mouse retina and brain . In June 2022, Seth Blackshaw’s team published some experimental results in the journal Cell Reports [5] .
It is worth mentioning that in February 2023, Yang Hui’s team published a paper titled: Ptbp1 knockdown failed to induce astrocytes to neurons in vivo in the journal Gene Therapy [6] .
This study indicated that Cas13x-mediated knockdown of Ptbp1 failed to induce glial-to-neuron conversion in vivo.
Thus negating the results previously published in Cell , it was believed that the previous results were due to the leakage of GFAP-AAV.
On June 7, 2023, the team of Seth Blackshaw of the Johns Hopkins University School of Medicine published an article in Nature entitled: Ptbp1 deletion does not induce astrocyte-toneuron conversion [7] .
This study showed that Ptbp1 loss-of-function did not induce astrocyte-to-neuron differentiation, and did not even cause substantial changes in astrocyte gene expression.

Seth Blackshaw’s team pointed out that the conclusion of Ptbp1 knockdown-induced glial cell-to-neuron transdifferentiation observed in this Nature paper by Fu Xiangdong’s team lacks some necessary direct evidence.
Genetic lineage and single-cell RNA-sequencing (scRNA-Seq) analysis of mature astrocytes carrying heterozygous or homozygous mutations in Ptbp1 revealed that Ptbp1-deficient astrocytes did not transform into neurons, nor did they Substantial effects on expression of other genes.
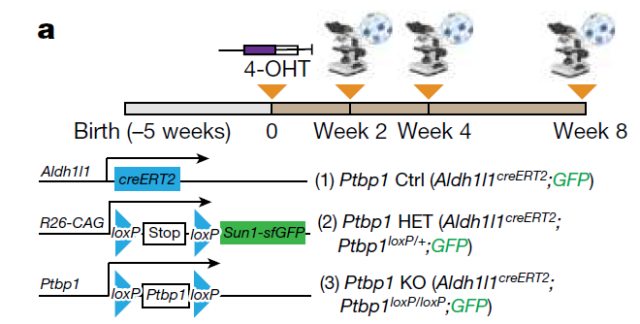
To genetically disrupt Ptbp1 function in astrocytes, the research team used astrocyte-specific tamoxifen-induced Aldh1l1 creERT2 mice and Sun1-GFP loxP/loxP and Ptbp1 loxP/loxP cell lines , and used 4-hydroxytamoxifen (4-OHT) to induce heterozygous or homozygous Ptbp1 deletion in astrocytes that were irreversibly marked by SUN1-GFP for lineage tracing.
The conversion of novel glial cells to neurons in the brain was not observed in mice of any genotype at 2, 4, and 8 weeks after 4-OHT induction.
The Nature paper by Fu Xiangdong’s team showed that neurons transformed from glial cells induced by knockdown of Ptbp1 can secrete dopamine and restore the Parkinson’s disease mouse model induced by 6-hydroxydopamine (6-OHDA) -damaged dopaminergic neurons motor function.
However, this questioning article published in Nature showed that neither mice with heterozygous or homozygous deletion of Ptbp1 damaged by 6-OHDA developed new neurons.
Therefore, the research team concluded that Ptbp1 loss-of-function does not lead to astrocyte-to-neuron conversion, with or without neuronal damage.
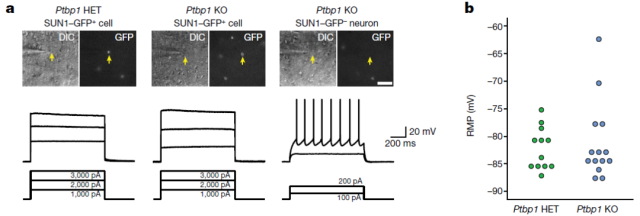
Next, the research team examined whether loss of Ptbp1 altered the physiological activity of astrocytes. Patch-clamp experiments revealed that neither heterozygous nor homozygous deletion of Ptbp1 induced neuron-like electrophysiological properties in astrocytes.
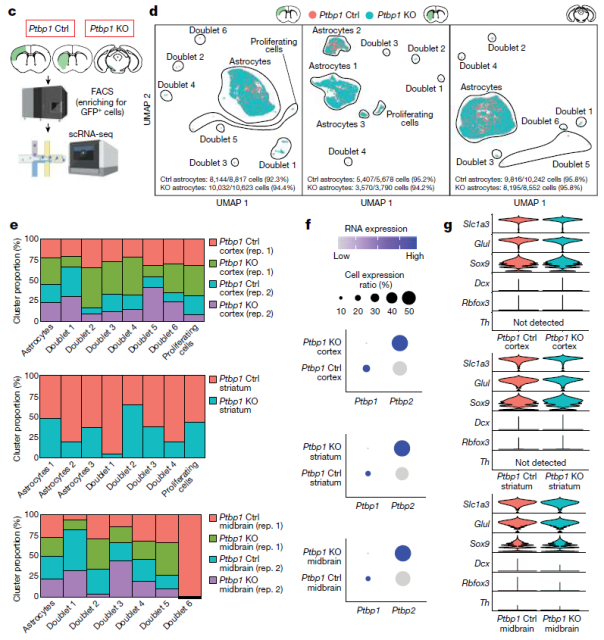
In order to comprehensively analyze the molecular effects of Ptbp1 deletion in novel glial cells, the research team analyzed the whole cortex, striatum and substantia nigra of wild-type, heterozygous and homozygous Ptbp1-null mice after 2 weeks of 4-OHT induction. Single-cell RNA sequencing (scRNA-seq) analysis was performed .
The results showed that the expression of most astrocyte markers was unchanged after Ptbp1 deletion, and no induction of neural progenitor or mature neuron-specific genes was observed.
Immunostaining experiments confirmed that Ptbp1-deleted astrocytes retained expression of the glial marker SOX9. The research team concluded that Ptbp1 does not suppress the expression of neuronal genes in astrocytes.
To further study the gene expression changes induced by loss of Ptbp1 function at later time points, our research team induced GFP-positive astrocytes and fluorescence-activated cells in wild-type and homozygous Ptbp1-null mice for 4 weeks after 4-OHT induction. The enriched astrocyte-derived cells were sorted for further scRNA-seq analysis.
The results showed no differences in the expression of neurogenic or other cell type-specific marker genes in all three brain regions following Ptbp1 loss.
Expression of most astrocyte markers was unchanged, and no induction of neural progenitor or mature neuron-specific genes was observed.
These results indicated that astrocytes after deletion of Ptbp1 did not exhibit glial cell-specific gene expression, nor did astrocyte-specific gene expression be down-regulated.
Overall, this study achieved efficient and cell-specific knockout of Ptbp1, but no astrocyte-to-neuron transformation was observed in either Ptbp1 heterozygous or homozygous deletion mice.
Single-cell RNA sequencing (scRNA-seq) analysis revealed subtle changes in gene expression in heterozygous and homozygous Ptbp1-deficient astrocytes, but did not induce neuron-specific gene expression or neuron-like electrophysiological properties.
Combined with a series of recently published papers that failed to replicate the results of knocking down Ptbp1 to achieve the transformation of glial cells into neurons, and Yang Hui’s team’s denial of the results of their previous Cell papers, the research team stated that after knocking down Ptbp1, glial cells It is unlikely that the result of the conversion of cells to neurons is caused by loss of function of Ptbp1.
This also highlights the importance of using genetic manipulation, lineage tracing, and gene expression analysis in the study of cell type conversion.
Judging from the submission records of the paper, this paper was submitted to Nature in September 2021 , and a large number of experiments and data have since been supplemented, thus more rigorously discussing the point that the loss of Ptbp1 function cannot induce the transformation of glial cells into neurons .
Professor Zhang Chunli , who was the first to question the role of Ptbp1 through the paper, said bluntly: Even if the inhibition of Ptbp1 has therapeutic potential, it does not stem from the transformation of glial cells into neurons.
In January this year, Professor Zhang Chunli published a review paper titled: Therapeutic Potential of PTBP1 Inhibition, If Any, Is Not Attributed to Glia-to-Neuron Conversion in the Annual Review of Neuroscience [7] .
In this review, Professor Zhang Chunli reviewed the research controversies of Ptbp1-based glial cell-to-neuron conversion, and summarized suggestions for future research on glial cell-to-neuron conversion in vivo and Ptbp1.
Image

For the questioning article published by Seth Blackshaw’s team in Nature , Fu Xiangdong’s team published a response in Nature at the same time [8] .
Fu Xiangdong’s team said that after analyzing the single-cell RNA sequencing (scRNA-seq) data released by Seth Blackshaw’s team, they came to the opposite conclusion.
In addition, previous studies in many biological systems have shown that knockout versus knockdown results in different phenotypes, therefore, knockout and knockdown of Ptbp1 may produce different phenotypes.
While it cannot be ruled out that the observed conversion of astrocytes to neurons may be due to leakage from existing neurons, the current scRNA-seq and immunostaining data suggest the presence of an astrocyte or astrocyte The possibility of a subset of morphocyte-like cells that are more prone to redirection to the neuronal lineage than mature astrocytes awaits future investigation.

Paper link :
1. https://doi.org/10.1016/j.cell.2020.03.024
2. https://www.nature.com/articles/s41586-020-2388-4
3. https://doi.org/10.1016/j.cell.2021.09.005
4. https://doi.org/10.1101/2021.10.04.462784
5. https://doi.org/10.1016/j.celrep.2022.110849
6. https://www.nature.com/articles/s41434-023-00382-5
7. https://doi.org/10.1146/annurev-neuro-092822-083410
8. https://www.nature.com/articles/s41586-023-06067-8
Famous papers questioned: The loss of Ptbp1 could not induce the transformation of glial cells into neurons.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



