AstraZeneca COVID-19 vaccine seems to be inferior to Pfizer’s vaccine?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
AstraZeneca COVID-19 vaccine seems to be inferior to Pfizer’s vaccine?
AstraZeneca COVID-19 vaccine seems to be inferior to Pfizer’s vaccine? Lancet: Scotland data shows that the AstraZeneca COVID-19 vaccine seems to be inferior to Pfizer’s vaccine
Similar changes were observed for the Pfizer-BioNTech vaccine at least 14 days after the second vaccination.
On June 14, “The Lancet” published an online communication article stating that the variant B 1.617.2 from India quickly became the dominant strain of SARS-CoV-2 in Scotland. Alpha VOC (previously known as Kent VOC, B.1.1.7 or S gene negative) was the previous dominant strain, but it has been quickly replaced.
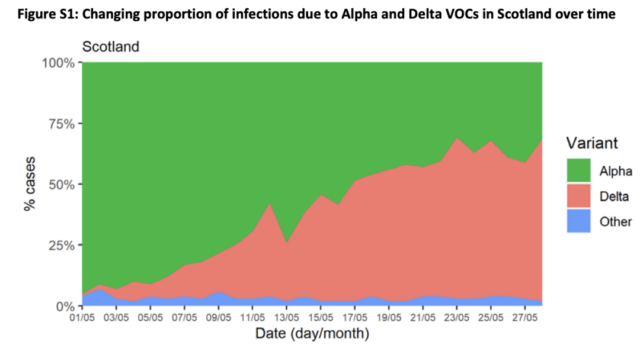
The Scottish Public Health Agency conducted a cohort analysis on the sequencing data of the Scottish COVID-19 surveillance platform EAVE II from April 1, 2021 to May 28, 2021 and found that 97% of the positive cases of the S gene sequenced in Scotland were Delta Variants, and 99% of Delta variants are positive for the S gene. And found that the AZ COVID-19 vaccine seems to be less effective than Pfizer’s COVID-19 vaccine. Compared with the alpha (B.1.1.7) variant, the number of hospitalizations has doubled.
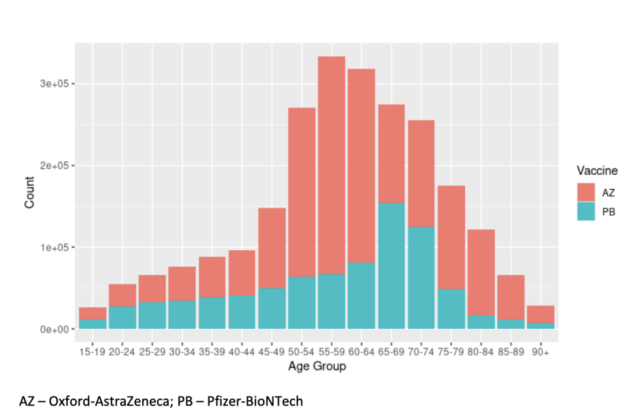
In general, the strong vaccine effect is not obvious until at least 28 days after the first vaccination (HR 0.32, 95% CI 0.22-0.46; Appendix p 3). In S gene negative cases, the effect of vaccination (at least 28 days after the first or second vaccination) is to reduce the risk of hospitalization (HR 0·28, 95% CI 0·18– 0·43). The corresponding hazard ratio for the admission risk of S gene positive cases is 0.38 (95% CI 0.24-0.58), the cross-test p-value is 0.19, and there is no evidence that the vaccine is positive for the first test Different impacts of hospitalization.
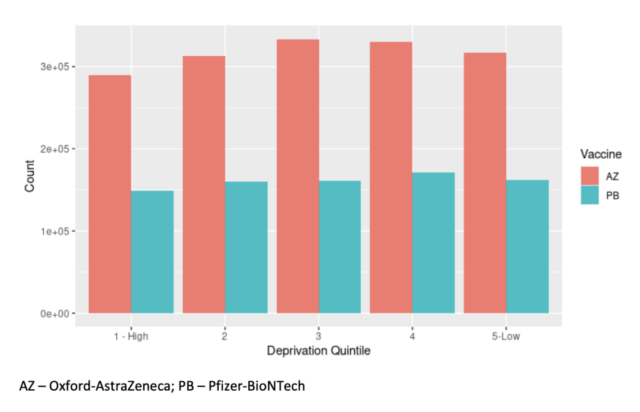
Considering the entire population (not just hospital cases), a negative test to evaluate the effectiveness of the vaccine in preventing SARS-CoV-2 infection confirmed by RT-PCR showed that the second At least 14 days after infection, BNT162b2 (Pfizer-BioNTech vaccine) provided very good protection: 92% (95% CI 90-93) S gene negative, 79% (75-82) S gene positive. However, the protective effect associated with ChAdOx1 nCoV-19 (Oxford-AstraZeneca vaccine) was significant but reduced.
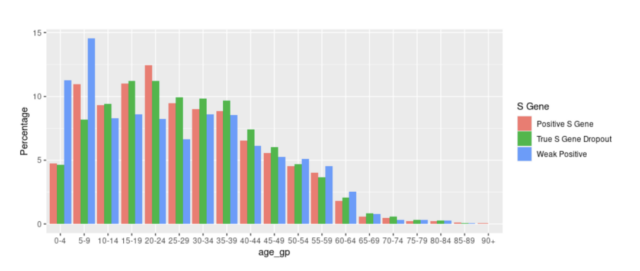
The S gene negative cases were 73% (95% CI 66-78), and those S gene were 60% (53-66)-positive. These estimates were obtained from a generalized additive logic model, which was adjusted for age, the time trend when swabs were collected, and the number of previous tests using splines plus gender and deprivation. Due to the vaccination trend and the increasing trend of Delta variants, the time adjustment of the overall trend may not fully explain these changes. In addition, no formal significance test was performed to compare vaccines.
Similar changes were observed for the Pfizer-BioNTech vaccine at least 14 days after the second vaccination, limiting the analysis to the population who reported symptoms at the time of the test, but the confidence interval associated with the reduction in sample size was wider. For the Oxford-AstraZeneca vaccine, as the S gene-negative vaccine is more effective, the changes are greater. These results are consistent with the effectiveness of the Delta VOC vaccine published by Public Health England.
However, it needs to be pointed out that doubling the number of hospitalizations of COVID-19 cases compared with the alpha variant does not mean that the Delta variant is more pathogenic. This can be done by increasing its infectivity by 60%.
Reference: SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness, Published: June 14, 2021 DOI: https://doi.org/10.1016/S0140-6736(21)01358-1
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



