Will China and India produce cheaper “Miracle Weight Loss Drug”Semaglutide soon?
- Major Breakthrough in Infertility Research: This Stem Cell Could Be Key to IVF and Other Fertility Treatments
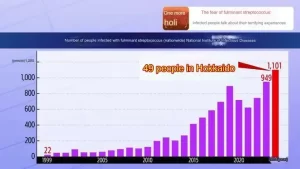
- Flesh-Eating Bacteria Infection Over 1000 Cases in Japan!
- CDC Recommends Updated COVID-19 Vaccines for 2024-2025 Season
- Will China and India produce cheaper “Miracle Weight Loss Drug”Semaglutide soon?
- Keto Diet Accelerates Aging and Promotes Cancer Metastasis
- The Critical Role of Immune Cell Triumvirates in Enhancing CD8+ T Cell Function
Will China and India produce cheaper “Miracle Weight Loss Drug”Semaglutide soon?
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- How serious is Japan’s “flesh-eating bacteria” problem?
- Taiwan 6th wave of COVID outbreak: 623 confirmed cases in one week and 38 deaths
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Will China and India produce cheaper “Miracle Weight Loss Drug”Semaglutide soon?
“Miracle Weight Loss Drug” Semaglutide Patent to Expire Soon: Cheaper Alternatives from China and India on the Horizon.
In recent years, semaglutide has taken the world by storm. This glucagon-like peptide-1 (GLP-1) receptor agonist has revolutionized weight loss, making it simpler than ever. Last year, this drug generated over $20 billion in sales, boosting the stock price of its developer, Novo Nordisk, whose market value now exceeds $630 billion.
Due to the efforts of numerous pharmaceutical companies in China and India, semaglutide and other major weight loss drugs may soon become more affordable, benefiting a broader population.
A February 2024 report in The Lancet revealed that the global obesity population has reached 1 billion. As the two most populous countries, China and India have hundreds of millions of overweight and obese individuals, driving strong demand for weight loss drugs.
Mimicking Hormones for Effective Weight Loss
In the past few years, a new class of weight loss drugs has gained worldwide popularity. These drugs work by mimicking a naturally occurring hormone, GLP-1, which not only regulates blood sugar levels but also binds to receptors in the brain that control appetite and in the gut to slow digestion and create a feeling of fullness.
In 2014, the U.S. Food and Drug Administration (FDA) approved the first GLP-1 drug for weight loss, liraglutide. This synthetic acylated GLP-1 analogue has over 97% sequence similarity to the human GLP-1 and requires daily subcutaneous injections, resulting in an average weight loss of 8% over a year.
However, the weekly dosage and more significant weight loss effects of semaglutide, which was FDA-approved for weight loss in 2021, made it a global sensation. Semaglutide requires just one weekly injection and can reduce body weight by an average of 15%.
In 2023, the FDA approved tirzepatide, the first dual GLP-1 and GIP receptor agonist, for weight loss. This drug, also administered weekly, can lead to an average weight reduction of 22.5%.
These GLP-1 drugs not only aid in weight loss and type 2 diabetes management but also lower the risk of kidney failure, suppress inflammation, prevent serious cardiovascular events, and alleviate sleep apnea.
Generics from China and India Ready to Enter the Market
While effective, GLP-1 drugs come with a hefty price tag. In the U.S., monthly costs for semaglutide or tirzepatide can exceed $1,000.
The patents for liraglutide have already expired in China, and semaglutide’s patents will expire in China and India by 2026. Companies from these countries plan to significantly reduce the high prices of GLP-1 drugs through the development of biosimilars.
While the exact cost reductions of these biosimilars remain to be seen, prices could potentially drop to half or even one-tenth of the original drugs. Lower prices could increase accessibility and demand, further driving competition and reducing costs.
India has not yet approved any weight loss biosimilars, but Mumbai-based Glenmark Pharmaceuticals launched Lirafit, a liraglutide biosimilar for type 2 diabetes, in January. This drug costs around $1.20 per day, 70% cheaper than liraglutide. Additionally, Indian generics giant Biocon is developing liraglutide and semaglutide biosimilars, awaiting market approval.
In China, the National Medical Products Administration (NMPA) has approved two GLP-1 drugs for weight loss from domestic companies. Huadong Medicine produces a liraglutide biosimilar (brand name: Lirupin), and Gan & Lee Pharmaceuticals produces a fully human GLP-1 drug, benaglutide (brand name: Fisepin), which is identical to human GLP-1 and requires three daily injections due to its short half-life.
Several Chinese companies are preparing to enter the market when semaglutide’s patent expires in 2026. Six companies have initiated Phase 3 clinical trials for semaglutide biosimilars. Jiuyuan Gene’s semaglutide biosimilar has completed Phase 3 trials for type 2 diabetes and is likely to be the first to enter the Chinese market. The company has also started Phase 3 trials for its weight loss application.
With the arrival of semaglutide biosimilars, the weight loss market is set for a major shift, intensifying competition.
Beyond Generics: New Innovations
Besides biosimilars, pharmaceutical companies in India and China are developing new weight loss drugs. Sun Pharmaceuticals, based in Mumbai, is working on GL0034, a molecule with a slightly modified chemical structure compared to semaglutide, aiming to produce a cheaper yet equally effective weight loss drug. Early clinical trials show that high-dose users lost 10% of their body weight in two months. The company is also developing a new dual-target weight loss drug to compete with tirzepatide.
In China, Innovent Biologics is collaborating with Eli Lilly to develop mazdutide, a dual GLP-1/GCG receptor agonist. GCG aids in metabolism and fat burning. Phase 2 trials showed that mazdutide’s weight loss effects are comparable to semaglutide, with a 9 mg dose group achieving a 15.4% weight reduction in Chinese obese patients with an average BMI of 34.3 after 24 weeks. Next week, Innovent will present Phase 3 trial data at the American Diabetes Association annual meeting, with reports indicating a 19% weight reduction in the 9 mg dose group after nearly a year.
Mazdutide is expected to receive regulatory approval for weight loss in China by early 2025, potentially becoming the first GLP-1/GCG dual-target weight loss drug approved globally.
Will China and India produce cheaper “Miracle Weight Loss Drug”Semaglutide soon?
References:
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)02750-2/fulltex
thttps://www.nature.com/articles/d41586-024-02044-x
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.