FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA has mandated a top-level black box warning for all marketed CAR-T therapies, citing a potential risk of inducing malignant T-cell tumors.
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA has mandated a top-level black box warning for all marketed CAR-T therapies, citing a potential risk of inducing malignant T-cell tumors.
Over the past decade, CAR-T cell therapy has transformed the field of oncology, providing a cure for previously incurable hematologic cancers.
In 2010, Professor Carl June pioneered the advancement of CAR-T cell therapy into human clinical trials, successfully “curing” several leukemia patients.
In 2017, the FDA granted its first approval for CAR-T therapy, and since then, six CAR-T therapies have been approved for treating blood cancers such as leukemia and lymphoma.
The success of CAR-T therapy has rekindled hope for cancer patients awaiting bone marrow matches and heralded the era of cell-based treatments.
CAR-T cell therapy, or Chimeric Antigen Receptor T-cell immunotherapy, involves engineering a patient’s immune T-cells outside the body to recognize antigens on the surface of tumor cells.
These modified cells are then infused back into the patient, effectively identifying and killing cancer cells.
Scientists have been actively exploring the application of CAR-T therapy beyond hematologic cancers, showing potential in solid tumors, autoimmune diseases, chronic infections, heart disease, and age-related conditions, according to clinical and preclinical studies.
In late November 2023, the U.S. FDA announced an investigation into a severe risk of T-cell malignancies in patients undergoing autologous CAR-T cell immunotherapy targeting BCMA or CD19.
On January 22, 2024, the FDA mandated a black box warning for the prescription labels of six marketed CAR-T cell therapies, cautioning patients undergoing autologous CAR-T cell immunotherapy targeting BCMA or CD19 about the risk of T-cell malignancies. A black box warning represents the highest level of drug adverse reaction warning required by the FDA, signifying significant risks of severe, potentially life-threatening adverse reactions associated with the drug.
Prior to this, the FDA had received reports of T-cell malignancies, including lymphomas and leukemias, in patients treated with autologous CAR-T cell immunotherapy targeting BCMA or CD19, from both clinical trials and adverse event data of marketed CAR-T cell therapies.
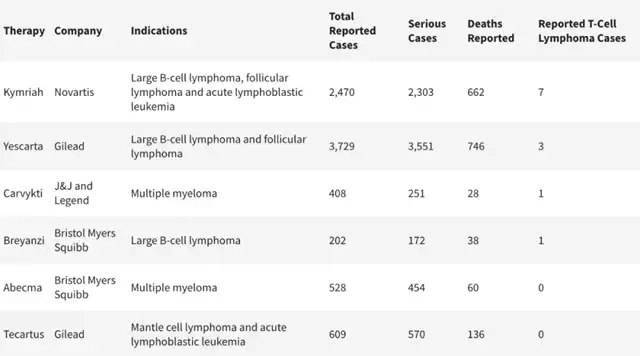
The FDA has identified the risk of T-cell malignancies as applicable to all currently approved autologous CAR-T cell immunotherapies targeting BCMA or CD19. While T-cell malignancies have occurred in patients treated with these products, current analyses have not provided evidence of a causal relationship between CAR-T cell therapy and T-cell malignancies.
The FDA advises ongoing monitoring for new malignancies in patients and clinical trial participants receiving CAR-T cell therapy. In case of new malignancies post-treatment, the FDA recommends reporting the adverse event to the developing company and obtaining patient samples for detecting the chimeric antigen receptor (CAR) transgene.
Novartis, along with Johnson & Johnson and Legend Biotech, has committed to updating the prescription information for their respective CAR-T cell therapies Kymriah (developed by Novartis) and Carvykti (developed by Johnson & Johnson and Legend Biotech) to include the risk of T-cell malignancies associated with CAR-T therapy.
Novartis also stated that, among over 10,000 patients treated with Kymriah, no causal relationship between the treatment and secondary malignancies has been identified, maintaining confidence in the therapy’s prospects.
Bristol Myers Squibb stated that it is currently evaluating the next steps regarding the addition of a black box warning to its two CAR-T cell therapies, Abecma and Breyanzi. No reports of secondary malignancies have been received from over 5,000 patients treated with these therapies, and no causal relationship with malignancies has been observed.
Gilead Sciences, the developer of Tecartus and Yescarta CAR-T therapies, expressed confidence in the overall safety of its products. These therapies have treated over 17,700 cancer patients, and there is currently no evidence suggesting a causal relationship between their use and the development of new malignancies. Gilead maintains stringent monitoring procedures and will fully cooperate with the FDA in analyzing data related to this investigation.
In summary, the FDA believes that the benefits of these CAR-T cell therapies still outweigh the potential risks, and while investigating the risk of T-cell malignancies related to treatment, the FDA does not intend to withdraw approval for these six CAR-T cell therapies.
The potential cancer risk associated with CAR-T cell therapy is believed to stem from the use of gene delivery vectors, specifically retroviral vectors. These vectors integrate their genetic material into the host cell genome, persisting with cell division, ensuring treatment stability. However, if the insertion site is near DNA sequences associated with cancer, there is a potential risk of inducing malignancies.
FDA has mandated a top-level black box warning for all marketed CAR-T therapies, citing a potential risk of inducing malignant T-cell tumors.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



